sRNA-Effector: A tool to expedite discovery of small RNA regulators
- PMID: 38469560
- PMCID: PMC10926228
- DOI: 10.1016/j.isci.2024.109300
sRNA-Effector: A tool to expedite discovery of small RNA regulators
Abstract
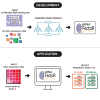
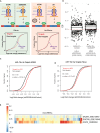
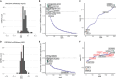
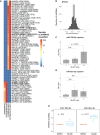
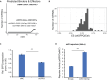
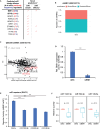
microRNAs (miRNAs) are small regulatory RNAs that repress target mRNA transcripts through base pairing. Although the mechanisms of miRNA production and function are clearly established, new insights into miRNA regulation or miRNA-mediated gene silencing are still emerging. In order to facilitate the discovery of miRNA regulators or effectors, we have developed sRNA-Effector, a machine learning algorithm trained on enhanced crosslinking and immunoprecipitation sequencing and RNA sequencing data following knockdown of specific genes. sRNA-Effector can accurately identify known miRNA biogenesis and effector proteins and identifies 9 putative regulators of miRNA function, including serine/threonine kinase STK33, splicing factor SFPQ, and proto-oncogene BMI1. We validated the role of STK33, SFPQ, and BMI1 in miRNA regulation, showing that sRNA-Effector is useful for identifying new players in small RNA biology. sRNA-Effector will be a web tool available for all researchers to identify potential miRNA regulators in any cell line of interest.
Keywords: Biocomputational method; Gene network; Machine learning; Nucleic acids.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function.PLoS Genet. 2015 Aug 26;11(8):e1005475. doi: 10.1371/journal.pgen.1005475. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26308709 Free PMC article.
-
Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA.PLoS One. 2012;7(9):e44873. doi: 10.1371/journal.pone.0044873. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984580 Free PMC article.
-
A survey of software tools for microRNA discovery and characterization using RNA-seq.Brief Bioinform. 2019 May 21;20(3):918-930. doi: 10.1093/bib/bbx148. Brief Bioinform. 2019. PMID: 29126230
-
Plant microRNAs: biogenesis, gene silencing, web-based analysis tools and their use as molecular markers.3 Biotech. 2019 Nov;9(11):413. doi: 10.1007/s13205-019-1942-y. Epub 2019 Oct 23. 3 Biotech. 2019. PMID: 31696018 Free PMC article. Review.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

