Loss of FoxO1 activates an alternate mechanism of mitochondrial quality control for healthy adipose browning
- PMID: 38469619
- PMCID: PMC10932742
- DOI: 10.1042/CS20230973
Loss of FoxO1 activates an alternate mechanism of mitochondrial quality control for healthy adipose browning
Abstract
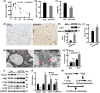
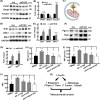
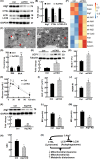
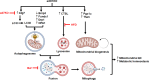
Browning of white adipose tissue is hallmarked by increased mitochondrial density and metabolic improvements. However, it remains largely unknown how mitochondrial turnover and quality control are regulated during adipose browning. In the present study, we found that mice lacking adipocyte FoxO1, a transcription factor that regulates autophagy, adopted an alternate mechanism of mitophagy to maintain mitochondrial turnover and quality control during adipose browning. Post-developmental deletion of adipocyte FoxO1 (adO1KO) suppressed Bnip3 but activated Fundc1/Drp1/OPA1 cascade, concurrent with up-regulation of Atg7 and CTSL. In addition, mitochondrial biogenesis was stimulated via the Pgc1α/Tfam pathway in adO1KO mice. These changes were associated with enhanced mitochondrial homeostasis and metabolic health (e.g., improved glucose tolerance and insulin sensitivity). By contrast, silencing Fundc1 or Pgc1α reversed the changes induced by silencing FoxO1, which impaired mitochondrial quality control and function. Ablation of Atg7 suppressed mitochondrial turnover and function, causing metabolic disorder (e.g., impaired glucose tolerance and insulin sensitivity), regardless of elevated markers of adipose browning. Consistently, suppression of autophagy via CTSL by high-fat diet was associated with a reversal of adO1KO-induced benefits. Our data reveal a unique role of FoxO1 in coordinating mitophagy receptors (Bnip3 and Fundc1) for a fine-tuned mitochondrial turnover and quality control, underscoring autophagic clearance of mitochondria as a prerequisite for healthy browning of adipose tissue.
Keywords: FoxO1; adipose browning; metabolism; mitochondrial quality control; mitophagy.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

