Wall teichoic acid substitution with glucose governs phage susceptibility of Staphylococcus epidermidis
- PMID: 38470054
- PMCID: PMC11005348
- DOI: 10.1128/mbio.01990-23
Wall teichoic acid substitution with glucose governs phage susceptibility of Staphylococcus epidermidis
Abstract
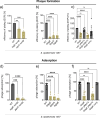
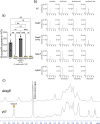
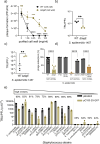
The species- and clone-specific susceptibility of Staphylococcus cells for bacteriophages is governed by the structures and glycosylation patterns of wall teichoic acid (WTA) glycopolymers. The glycosylation-dependent phage-WTA interactions in the opportunistic pathogen Staphylococcus epidermidis and in other coagulase-negative staphylococci (CoNS) have remained unknown. We report a new S. epidermidis WTA glycosyltransferase TagE whose deletion confers resistance to siphoviruses such as ΦE72 but enables binding of otherwise unbound podoviruses. S. epidermidis glycerolphosphate WTA was found to be modified with glucose in a tagE-dependent manner. TagE is encoded together with the enzymes PgcA and GtaB providing uridine diphosphate-activated glucose. ΦE72 transduced several other CoNS species encoding TagE homologs, suggesting that WTA glycosylation via TagE is a frequent trait among CoNS that permits interspecies horizontal gene transfer. Our study unravels a crucial mechanism of phage-Staphylococcus interaction and horizontal gene transfer, and it will help in the design of anti-staphylococcal phage therapies.IMPORTANCEPhages are highly specific for certain bacterial hosts, and some can transduce DNA even across species boundaries. How phages recognize cognate host cells remains incompletely understood. Phages infecting members of the genus Staphylococcus bind to wall teichoic acid (WTA) glycopolymers with highly variable structures and glycosylation patterns. How WTA is glycosylated in the opportunistic pathogen Staphylococcus epidermidis and in other coagulase-negative staphylococci (CoNS) species has remained unknown. We describe that S. epidermidis glycosylates its WTA backbone with glucose, and we identify a cluster of three genes responsible for glucose activation and transfer to WTA. Their inactivation strongly alters phage susceptibility patterns, yielding resistance to siphoviruses but susceptibility to podoviruses. Many different CoNS species with related glycosylation genes can exchange DNA via siphovirus ΦE72, suggesting that glucose-modified WTA is crucial for interspecies horizontal gene transfer. Our finding will help to develop antibacterial phage therapies and unravel routes of genetic exchange.
Keywords: Staphylococcus epidermidis; bacteriophages; coagulase-negative staphylococci; glycosyltransferase; wall teichoic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Genomic Analysis of the Unusual Staphylococcus aureus ST630 Isolates Harboring WTA Glycosyltransferase Genes tarM and tagN.Microbiol Spectr. 2022 Feb 23;10(1):e0150121. doi: 10.1128/spectrum.01501-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35170993 Free PMC article.
-
Biosynthesis of the unique wall teichoic acid of Staphylococcus aureus lineage ST395.mBio. 2014 Apr 8;5(2):e00869. doi: 10.1128/mBio.00869-14. mBio. 2014. PMID: 24713320 Free PMC article.
-
Staphylococcus epidermidis clones express Staphylococcus aureus-type wall teichoic acid to shift from a commensal to pathogen lifestyle.Nat Microbiol. 2021 Jun;6(6):757-768. doi: 10.1038/s41564-021-00913-z. Epub 2021 May 24. Nat Microbiol. 2021. PMID: 34031577
-
Phage susceptibility determinants of the opportunistic pathogen Staphylococcus epidermidis.Curr Opin Microbiol. 2024 Apr;78:102434. doi: 10.1016/j.mib.2024.102434. Epub 2024 Feb 16. Curr Opin Microbiol. 2024. PMID: 38364502 Review.
-
Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus.Int J Med Microbiol. 2014 May;304(3-4):215-21. doi: 10.1016/j.ijmm.2013.10.009. Epub 2013 Nov 1. Int J Med Microbiol. 2014. PMID: 24365646 Review.
Cited by
-
Carbohydrates in action: influencing infection and amplification of Staphylococcus aureus bacteriophages.BMC Microbiol. 2025 Aug 5;25(1):483. doi: 10.1186/s12866-025-04219-6. BMC Microbiol. 2025. PMID: 40764526 Free PMC article.
-
Bacteriophages from human skin infecting coagulase-negative Staphylococcus: diversity, novelty and host resistance.Sci Rep. 2024 Apr 8;14(1):8245. doi: 10.1038/s41598-024-59065-9. Sci Rep. 2024. PMID: 38589670 Free PMC article.
-
Intraspecies warfare restricts strain coexistence in human skin microbiomes.bioRxiv [Preprint]. 2025 Mar 11:2024.05.07.592803. doi: 10.1101/2024.05.07.592803. bioRxiv. 2025. Update in: Nat Microbiol. 2025 Jul;10(7):1581-1592. doi: 10.1038/s41564-025-02041-4. PMID: 38765968 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

