Growth modulatory effects of fenretinide encompass keratinocyte terminal differentiation: a favorable outcome for oral squamous cell carcinoma chemoprevention
- PMID: 38470060
- PMCID: PMC11519021
- DOI: 10.1093/carcin/bgae022
Growth modulatory effects of fenretinide encompass keratinocyte terminal differentiation: a favorable outcome for oral squamous cell carcinoma chemoprevention
Abstract
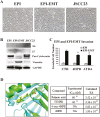
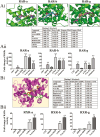
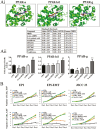
Oral squamous cell carcinoma (OSCC) is worldwide health problem associated with high morbidity and mortality. From both the patient and socioeconomic perspectives, prevention of progression of premalignant oral intraepithelial neoplasia (OIN) to OSCC is clearly the preferable outcome. Optimal OSCC chemopreventives possess a variety of attributes including high tolerability, bioavailability, efficacy and preservation of an intact surface epithelium. Terminal differentiation, which directs oral keratinocytes leave the proliferative pool to form protective cornified envelopes, preserves the protective epithelial barrier while concurrently eliminating growth-aberrant keratinocytes. This study employed human premalignant oral keratinocytes and an OSCC cell line to evaluate the differentiation-inducing capacity of the synthetic retinoid, fenretinide (4HPR). Full-thickness oral mucosal explants were evaluated for proof of concept differentiation studies. Results of this study characterize the ability of 4HPR to fulfill all requisite components for keratinocyte differentiation, i.e. nuclear import via binding to cellular RA binding protein-II (molecular modeling), binding to and subsequent activation of retinoic acid nuclear receptors (receptor activation assays), increased expression and translation of genes associated with keratinocyte differentiation [Reverse transcription polymerase chain reaction (RT-PCR), immunoblotting] upregulation of a transglutaminase enzyme essential for cornified envelope formation (transglutaminase 3, functional assay) and augmentation of terminal differentiation in human oral epithelial explants (image-analyses quantified corneocyte desquamation). These data build upon the chemoprevention repertoire of 4HPR that includes function as a small molecule kinase inhibitor and inhibition of essential mechanisms necessary for basement membrane invasion. An upcoming clinical trial, which will assess whether a 4HPR-releasing mucoadhesive patch induces histologic, clinical and molecular regression in OIN lesions, will provide essential clinical insights.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures


Similar articles
-
Fenretinide Perturbs Focal Adhesion Kinase in Premalignant and Malignant Human Oral Keratinocytes. Fenretinide's Chemopreventive Mechanisms Include ECM Interactions.Cancer Prev Res (Phila). 2015 May;8(5):419-30. doi: 10.1158/1940-6207.CAPR-14-0418. Epub 2015 Feb 24. Cancer Prev Res (Phila). 2015. PMID: 25712051 Free PMC article.
-
Evaluation of a mucoadhesive fenretinide patch for local intraoral delivery: a strategy to reintroduce fenretinide for oral cancer chemoprevention.Carcinogenesis. 2012 May;33(5):1098-105. doi: 10.1093/carcin/bgs122. Epub 2012 Mar 15. Carcinogenesis. 2012. PMID: 22427354 Free PMC article.
-
Fenretinide combines perturbation of signaling kinases, cell-extracellular matrix interactions and matrix metalloproteinase activation to inhibit invasion in oral squamous cell carcinoma cells.Carcinogenesis. 2022 Oct 22;43(9):851-864. doi: 10.1093/carcin/bgac070. Carcinogenesis. 2022. PMID: 35974187 Free PMC article.
-
Suppression of squamous cell carcinoma growth and differentiation by retinoids.Cancer Res. 1994 Apr 1;54(7 Suppl):1987s-1990s. Cancer Res. 1994. PMID: 8137325 Review.
-
Retinoids: application in premalignant lesions and oral cancer.Med Oral. 2001 Mar-Apr;6(2):114-23. Med Oral. 2001. PMID: 11500628 Review. English, Spanish.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

