Corrected and republished from: "Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence"
- PMID: 38470135
- PMCID: PMC11003230
- DOI: 10.1128/iai.00037-24
Corrected and republished from: "Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence"
Abstract
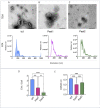
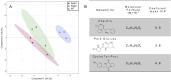
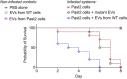
Small molecules are components of fungal extracellular vesicles (EVs), but their biological roles are only superficially known. NOP16 is a eukaryotic gene that is required for the activity of benzimidazoles against Cryptococcus deuterogattii. In this study, during the phenotypic characterization of C. deuterogattii mutants expected to lack NOP16 expression, we observed a reduced EV production. Whole-genome sequencing, RNA-Seq, and cellular proteomics revealed that, contrary to our initial findings, these mutants expressed Nop16 but exhibited altered expression of 14 genes potentially involved in sugar transport. Based on this observation, we designated these mutant strains as Past1 and Past2, representing
Keywords: Cryptococcus; extracellular vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Corrected and republished from
-
Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence and Requires the NOP16 Gene.Infect Immun. 2022 Aug 18;90(8):e0023222. doi: 10.1128/iai.00232-22. Epub 2022 Jul 12. Infect Immun. 2022. Corrected and republished in: Infect Immun. 2024 Apr 9;92(4):e0003724. doi: 10.1128/iai.00037-24. PMID: 35862719 Free PMC article. Corrected and republished.
Similar articles
-
Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence and Requires the NOP16 Gene.Infect Immun. 2022 Aug 18;90(8):e0023222. doi: 10.1128/iai.00232-22. Epub 2022 Jul 12. Infect Immun. 2022. Corrected and republished in: Infect Immun. 2024 Apr 9;92(4):e0003724. doi: 10.1128/iai.00037-24. PMID: 35862719 Free PMC article. Corrected and republished.
-
A Novel Protocol for the Isolation of Fungal Extracellular Vesicles Reveals the Participation of a Putative Scramblase in Polysaccharide Export and Capsule Construction in Cryptococcus gattii.mSphere. 2019 Mar 20;4(2):e00080-19. doi: 10.1128/mSphere.00080-19. mSphere. 2019. PMID: 30894430 Free PMC article.
-
Small Molecule Analysis of Extracellular Vesicles Produced by Cryptococcus gattii: Identification of a Tripeptide Controlling Cryptococcal Infection in an Invertebrate Host Model.Front Immunol. 2021 Mar 16;12:654574. doi: 10.3389/fimmu.2021.654574. eCollection 2021. Front Immunol. 2021. PMID: 33796117 Free PMC article.
-
Pathogenic Delivery: The Biological Roles of Cryptococcal Extracellular Vesicles.Pathogens. 2020 Sep 16;9(9):754. doi: 10.3390/pathogens9090754. Pathogens. 2020. PMID: 32948010 Free PMC article. Review.
-
Context-specific regulation of extracellular vesicle biogenesis and cargo selection.Nat Rev Mol Cell Biol. 2023 Jul;24(7):454-476. doi: 10.1038/s41580-023-00576-0. Epub 2023 Feb 10. Nat Rev Mol Cell Biol. 2023. PMID: 36765164 Free PMC article. Review.
Cited by
-
Fungal Extracellular Vesicle Proteins with Potential in Biological Interaction.Molecules. 2024 Aug 24;29(17):4012. doi: 10.3390/molecules29174012. Molecules. 2024. PMID: 39274860 Free PMC article. Review.
-
Things you wanted to know about fungal extracellular vesicles (but were afraid to ask).PLoS Negl Trop Dis. 2025 May 22;19(5):e0013038. doi: 10.1371/journal.pntd.0013038. eCollection 2025 May. PLoS Negl Trop Dis. 2025. PMID: 40403031 Free PMC article. No abstract available.
-
Hidden allies: how extracellular vesicles drive biofilm formation, stress adaptation, and host-immune interactions in human fungal pathogens.mBio. 2024 Dec 11;15(12):e0304523. doi: 10.1128/mbio.03045-23. Epub 2024 Nov 18. mBio. 2024. PMID: 39555918 Free PMC article. Review.
References
-
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59. doi:10.1128/EC.00318-06 - DOI - PMC - PubMed
-
- Rizzo J, Wong SSW, Gazi AD, Moyrand F, Chaze T, Commere P-H, Novault S, Matondo M, Péhau-Arnaudet G, Reis FCG, Vos M, Alves LR, May RC, Nimrichter L, Rodrigues ML, Aimanianda V, Janbon G. 2021. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J Extracell Vesicles 10:e12129. doi:10.1002/jev2.12129 - DOI - PMC - PubMed
Publication types
Grants and funding
- 440015/2018-9/Ministério da Saúde (Ministry of Health)
- 405520/2018-2, 301304/2017-3/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- PROEP-ICC 442186/2019-3, VPPCB-007-FIO-18, VPPIS-001-FIO18/Fundação Oswaldo Cruz (FIOCRUZ)
- 2021/00728-0/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- Finance Code 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
LinkOut - more resources
Full Text Sources
Miscellaneous

