Pyoluteorin regulates the biosynthesis of 2,4-DAPG through the TetR family transcription factor PhlH in Pseudomonas protegens Pf-5
- PMID: 38470180
- PMCID: PMC11022555
- DOI: 10.1128/aem.01743-23
Pyoluteorin regulates the biosynthesis of 2,4-DAPG through the TetR family transcription factor PhlH in Pseudomonas protegens Pf-5
Abstract
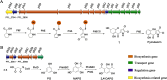
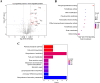
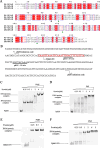
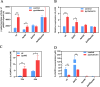
Soil and rhizosphere bacteria act as a rich source of secondary metabolites, effectively fighting against a diverse array of pathogens. Certain Pseudomonas species harbor biosynthetic gene clusters for producing both pyoluteorin and 2,4-diacetylphloroglucinol (2,4-DAPG), which are polyketides that exhibit highly similar antimicrobial spectrum against bacteria and fungi or oomycete. A complex cross talk exists between pyoluteorin and 2,4-DAPG biosynthesis, and production of 2,4-DAPG was strongly repressed by pyoluteorin, yet the underlying mechanism is still elusive. In this study, we find that the TetR family transcription factor PhlH is involved in the cross talk between pyoluteorin and 2,4-DAPG biosynthesis. PhlH binds to a palindromic sequence within the promoter of phlG (PphlG), which encodes a C-C bond hydrolase responsible for degrading 2,4-DAPG. As a signaling molecule, pyoluteorin disrupts the PhlH-PphlG complex by binding to PhlH, leading to decreased levels of 2,4-DAPG. Proteomics data suggest that pyoluteorin regulates multiple physiological processes including fatty acid biosynthesis and transportation of taurine, siderophore, and amino acids. Our work not only reveals a novel mechanism of cross talk between pyoluteorin and 2,4-DAPG biosynthesis, but also highlights pyoluteorin's role as a messenger in the complex communication network of Pseudomonas.IMPORTANCEAntibiosis serves as a crucial defense mechanism for microbes against invasive bacteria and resource competition. These bacteria typically orchestrate the production of multiple antibiotics in a coordinated fashion, wherein the synthesis of one antibiotic inhibits the generation of another. This strategic coordination allows the bacterium to focus its resources on producing the most advantageous antibiotic under specific circumstances. However, the underlying mechanisms of distinct antibiotic production in bacterial cells remain largely elusive. In this study, we reveal that the TetR family transcription factor PhlH detects the secondary metabolite pyoluteorin and mediates the cross talk between pyoluteorin and 2,4-DAPG biosynthesis in the biocontrol strain Pseudomonas protegens Pf-5. These findings hold promise for future research, potentially informing the manipulation of these systems to enhance the effectiveness of biocontrol agents.
Keywords: 2,4-DAPG; PhlH; Pseudomonas protegens; cross talk; pyoluteorin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. 2014. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 158:1402–1414. doi: 10.1016/j.cell.2014.08.032 - DOI - PMC - PubMed
-
- Skinnider MA, Johnston CW, Gunabalasingam M, Merwin NJ, Kieliszek AM, MacLellan RJ, Li H, Ranieri MRM, Webster ALH, Cao MPT, Pfeifle A, Spencer N, To QH, Wallace DP, Dejong CA, Magarvey NA. 2020. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat Commun 11:6058. doi: 10.1038/s41467-020-19986-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

