Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut
- PMID: 38470269
- PMCID: PMC11210198
- DOI: 10.1128/mbio.00078-24
Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut
Abstract
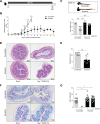
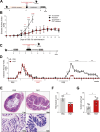
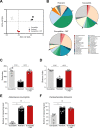
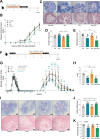
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the gastrointestinal tract. The etiology of IBD remains elusive, but the disease is suggested to arise from the interaction of environmental and genetic factors that trigger inadequate immune responses and inflammation in the intestine. The gut microbiome majorly contributes to disease as an environmental variable, and although some causative bacteria are identified, little is known about which specific members of the microbiome aid in the intestinal epithelial barrier function to protect from disease. While chemically inducing colitis in mice from two distinct animal facilities, we serendipitously found that mice in one facility showed remarkable resistance to disease development, which was associated with increased markers of epithelial barrier integrity. Importantly, we show that Akkermansia muciniphila and Parabacteroides distasonis were significantly increased in the microbiota of resistant mice. To causally connect these microbes to protection against disease, we colonized susceptible mice with the two bacterial species. Our results demonstrate that A. muciniphila and P. distasonis synergistically drive a protective effect in both acute and chronic models of colitis by boosting the frequency of type 3 innate lymphoid cells in the colon and by improving gut epithelial integrity. Altogether, our work reveals a combined effort of commensal microbes in offering protection against severe intestinal inflammation by shaping gut immunity and by enhancing intestinal epithelial barrier stability. Our study highlights the beneficial role of gut bacteria in dictating intestinal homeostasis, which is an important step toward employing microbiome-driven therapeutic approaches for IBD clinical management.
Importance: The contribution of the gut microbiome to the balance between homeostasis and inflammation is widely known. Nevertheless, the etiology of inflammatory bowel disease, which is known to be influenced by genetics, immune response, and environmental cues, remains unclear. Unlocking novel players involved in the dictation of a protective gut, namely, in the microbiota component, is therefore crucial to develop novel strategies to tackle IBD. Herein, we revealed a synergistic interaction between two commensal bacterial strains, Akkermansia muciniphila and Parabacteroides distasonis, which induce protection against both acute and chronic models of colitis induction, by enhancing epithelial barrier integrity and promoting group 3 innate lymphoid cells in the colonic mucosa. This study provides a novel insight on how commensal bacteria can beneficially act to promote intestinal homeostasis, which may open new avenues toward the use of microbiome-derived strategies to tackle IBD.
Keywords: Akkermansia muciniphila; ILC; Parabacteroides distasonis; colitis; gut immunity; microbiome.
Conflict of interest statement
Mahesh S. Desai works as a consultant and an advisory board member at Theralution GmbH, Germany. The other authors declare no conflict of interest.
Figures

Similar articles
-
Akkermansia muciniphila and Parabacteroides distasonis as prognostic markers for relapse in ulcerative colitis patients.Front Cell Infect Microbiol. 2024 Jul 4;14:1367998. doi: 10.3389/fcimb.2024.1367998. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39027140 Free PMC article.
-
In Vitro Characterization of Gut Microbiota-Derived Commensal Strains: Selection of Parabacteroides distasonis Strains Alleviating TNBS-Induced Colitis in Mice.Cells. 2020 Sep 16;9(9):2104. doi: 10.3390/cells9092104. Cells. 2020. PMID: 32947881 Free PMC article.
-
Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis.PLoS One. 2013 Oct 24;8(10):e76520. doi: 10.1371/journal.pone.0076520. eCollection 2013. PLoS One. 2013. PMID: 24204633 Free PMC article.
-
The potential of Akkermansia muciniphila in inflammatory bowel disease.Appl Microbiol Biotechnol. 2021 Aug;105(14-15):5785-5794. doi: 10.1007/s00253-021-11453-1. Epub 2021 Jul 27. Appl Microbiol Biotechnol. 2021. PMID: 34312713 Review.
-
The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives.Front Immunol. 2023 Jan 6;13:1089600. doi: 10.3389/fimmu.2022.1089600. eCollection 2022. Front Immunol. 2023. PMID: 36685588 Free PMC article. Review.
Cited by
-
Akkermansia muciniphila and Parabacteroides distasonis as prognostic markers for relapse in ulcerative colitis patients.Front Cell Infect Microbiol. 2024 Jul 4;14:1367998. doi: 10.3389/fcimb.2024.1367998. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39027140 Free PMC article.
-
Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice.Microbiome. 2024 Dec 30;12(1):275. doi: 10.1186/s40168-024-01995-7. Microbiome. 2024. PMID: 39734222 Free PMC article.
-
Parabacteroides as a promising target for disease intervention: current stage and pending issues.NPJ Biofilms Microbiomes. 2025 Jul 19;11(1):137. doi: 10.1038/s41522-025-00772-0. NPJ Biofilms Microbiomes. 2025. PMID: 40681519 Free PMC article. Review.
-
Causal Effects of Gut Microbiome on Tinnitus: A Mendelian Randomization Study.J Multidiscip Healthc. 2025 Jul 22;18:4159-4172. doi: 10.2147/JMDH.S525502. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40727005 Free PMC article.
-
Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets.Animals (Basel). 2024 Jul 24;14(15):2156. doi: 10.3390/ani14152156. Animals (Basel). 2024. PMID: 39123683 Free PMC article.
References
-
- Pereira GV, Boudaud M, Wolter M, Alexander C, Sciscio AD, Grant E, Trindade BC, Pudlo NA, Singh S, Campbell A, Shan M, Zhang L, Yang Q, Willieme S, Kim K, Denike-Duval T, Fuentes J, Bleich A, Schmidt TM, Kennedy L, Lyssiotis CA, Chen GY, Eaton KA, Desai MS, Martens EC. 2023. Opposing diet, microbiome and metabolite mechanisms regulate inflammatory bowel disease in a genetically susceptible host. bioRxiv. doi:10.1101/2022.04.03.486886 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
- 10.54499/2020.00185.CEECIND/CP1600/CT0004/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 10.54499/CEECIND/04058/2018/CP1581/CT0015/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 2021.07836.BD/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 10.54499/CEECIND/04058/2018/CP1581/CT0015/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- C15/BM/10318186/Fonds National de la Recherche Luxembourg (FNR)
- C18/BM/12585940/Fonds National de la Recherche Luxembourg (FNR)
- PID2020-120292RB-I00/Ministerio de Ciencia e Innovación (MCIN)
- PCIN-2015-094/GVA | Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital, Generalitat Valenciana (CIUCSD)
- UIDB/50026/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- UIDP/50026/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- UIDB/04469/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 2021.06268.BD/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 10.54499/2021.01234.CEECIND/CP1664/CT0019/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- ESCMID Research Grant 2022/European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
- ECCO Grant 2023/European Crohn's and Colitis Organisation (ECCO)
- ECCO Pioneer Award 2022/European Crohn's and Colitis Organisation (ECCO)
- LA/P/0029/2020/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- 22/17426243/Fonds National de la Recherche Luxembourg (FNR)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

