Tailoring the Degradation Time of Polycationic PEG-Based Hydrogels toward Dynamic Cell Culture Matrices
- PMID: 38470448
- PMCID: PMC11022240
- DOI: 10.1021/acsabm.4c00057
Tailoring the Degradation Time of Polycationic PEG-Based Hydrogels toward Dynamic Cell Culture Matrices
Abstract
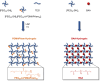
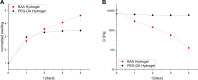
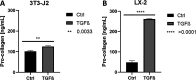
Poly(ethylene glycol)-based (PEG) hydrogels provide an ideal platform to obtain well-defined and tailor-made cell culture matrices to enhance in vitro cell culture conditions, although cell adhesion is often challenging when the cells are cultivated on the substrate surface. We herein demonstrate two approaches for the synthesis of polycationic PEG-based hydrogels which were modified to enhance cell-matrix interactions, to improve two-dimensional (2D) cell culture, and catalyze hydrolytic degradation. While the utilization of N,N-(bisacryloxyethyl) amine (BAA) as cross-linker for in situ gelation provides degradable scaffolds for dynamic cell culture, the incorporation of short segments of poly(N-(3-(dimethylamino)propyl)acrylamide) (PDMAPAam) provides high local cationic charge density leading to PEG-based hydrogels with high selectivity for fibroblastic cell lines. The adsorption of transforming growth factor (TGF-β) into the hydrogels induced stimulation of fibrosis and thus the formation of collagen as a natural ECM compound. With this, these dynamic hydrogels enhance in vitro cell culture by providing a well-defined, artificial, and degradable matrix that stimulates cells to produce their own natural scaffold within a defined time frame.
Keywords: 2D cell culture; degradable hydrogels; poly(ethylene glycol); polycationic hydrogel; transforming growth factor β.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor β3.Acta Biomater. 2019 Jul 15;93:97-110. doi: 10.1016/j.actbio.2019.03.046. Epub 2019 Mar 23. Acta Biomater. 2019. PMID: 30914256 Free PMC article.
-
Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering.Acta Biomater. 2016 Jul 15;39:1-11. doi: 10.1016/j.actbio.2016.05.015. Epub 2016 May 11. Acta Biomater. 2016. PMID: 27180026 Free PMC article.
-
Incorporation of a silicon-based polymer to PEG-DA templated hydrogel scaffolds for bioactivity and osteoinductivity.Acta Biomater. 2019 Nov;99:100-109. doi: 10.1016/j.actbio.2019.09.018. Epub 2019 Sep 16. Acta Biomater. 2019. PMID: 31536841 Free PMC article.
-
Biomaterials based on hyaluronic acid, collagen and peptides for three-dimensional cell culture and their application in stem cell differentiation.Int J Biol Macromol. 2023 Jan 31;226:14-36. doi: 10.1016/j.ijbiomac.2022.11.213. Epub 2022 Nov 24. Int J Biol Macromol. 2023. PMID: 36436602 Review.
-
Designing degradable hydrogels for orthogonal control of cell microenvironments.Chem Soc Rev. 2013 Sep 7;42(17):7335-72. doi: 10.1039/c3cs60040h. Epub 2013 Apr 22. Chem Soc Rev. 2013. PMID: 23609001 Free PMC article. Review.
Cited by
-
Biomaterials in Postoperative Adhesion Barriers and Uterine Tissue Engineering.Gels. 2025 Jun 9;11(6):441. doi: 10.3390/gels11060441. Gels. 2025. PMID: 40558740 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
