Neutrophil proteases are protective against SARS-CoV-2 by degrading the spike protein and dampening virus-mediated inflammation
- PMID: 38470488
- PMCID: PMC11128203
- DOI: 10.1172/jci.insight.174133
Neutrophil proteases are protective against SARS-CoV-2 by degrading the spike protein and dampening virus-mediated inflammation
Abstract
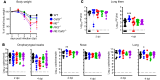
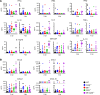
Studies on severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) have highlighted the crucial role of host proteases for viral replication and the immune response. The serine proteases furin and TMPRSS2 and lysosomal cysteine proteases facilitate viral entry by limited proteolytic processing of the spike (S) protein. While neutrophils are recruited to the lungs during COVID-19 pneumonia, little is known about the role of the neutrophil serine proteases (NSPs) cathepsin G (CatG), elastase (NE), and proteinase 3 (PR3) on SARS-CoV-2 entry and replication. Furthermore, the current paradigm is that NSPs may contribute to the pathogenesis of severe COVID-19. Here, we show that these proteases cleaved the S protein at multiple sites and abrogated viral entry and replication in vitro. In mouse models, CatG significantly inhibited viral replication in the lung. Importantly, lung inflammation and pathology were increased in mice deficient in NE and/or CatG. These results reveal that NSPs contribute to innate defenses against SARS-CoV-2 infection via proteolytic inactivation of the S protein and that NE and CatG limit lung inflammation in vivo. We conclude that therapeutic interventions aiming to reduce the activity of NSPs may interfere with viral clearance and inflammation in COVID-19 patients.
Keywords: COVID-19; Inflammation; Neutrophils; Proteases; Serpins.
Figures


Similar articles
-
The Proteolytic Activity of Neutrophil-Derived Serine Proteases Bound to the Cell Surface Arming Lung Epithelial Cells for Viral Defense.Molecules. 2024 Sep 19;29(18):4449. doi: 10.3390/molecules29184449. Molecules. 2024. PMID: 39339444 Free PMC article.
-
Camostat Does Not Inhibit the Proteolytic Activity of Neutrophil Serine Proteases.Pharmaceuticals (Basel). 2022 Apr 20;15(5):500. doi: 10.3390/ph15050500. Pharmaceuticals (Basel). 2022. PMID: 35631327 Free PMC article.
-
Genome-wide bioinformatics analysis of human protease capacity for proteolytic cleavage of the SARS-CoV-2 spike glycoprotein.Microbiol Spectr. 2024 Feb 6;12(2):e0353023. doi: 10.1128/spectrum.03530-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189333 Free PMC article.
-
Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets?Front Immunol. 2020 May 28;11:1229. doi: 10.3389/fimmu.2020.01229. eCollection 2020. Front Immunol. 2020. PMID: 32574272 Free PMC article. Review.
-
Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis - with impact on gastrointestinal tract.World J Gastroenterol. 2021 Oct 21;27(39):6590-6600. doi: 10.3748/wjg.v27.i39.6590. World J Gastroenterol. 2021. PMID: 34754154 Free PMC article. Review.
Cited by
-
Neutrophils Display Novel Partners of Cytosolic Proliferating Cell Nuclear Antigen Involved in Interferon Response in COVID-19 Patients.J Innate Immun. 2025;17(1):154-175. doi: 10.1159/000543633. Epub 2025 Feb 27. J Innate Immun. 2025. PMID: 40015257 Free PMC article.
-
Synergistic activation of bat SARS-like coronaviruses spike protein by elastase and TMPRSS2.Sci Rep. 2025 Jul 21;15(1):26469. doi: 10.1038/s41598-025-11600-y. Sci Rep. 2025. PMID: 40691229 Free PMC article.
-
The Proteolytic Activity of Neutrophil-Derived Serine Proteases Bound to the Cell Surface Arming Lung Epithelial Cells for Viral Defense.Molecules. 2024 Sep 19;29(18):4449. doi: 10.3390/molecules29184449. Molecules. 2024. PMID: 39339444 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

