P2Y13 receptor deficiency favors adipose tissue lipolysis and worsens insulin resistance and fatty liver disease
- PMID: 38470490
- PMCID: PMC11141875
- DOI: 10.1172/jci.insight.175623
P2Y13 receptor deficiency favors adipose tissue lipolysis and worsens insulin resistance and fatty liver disease
Abstract
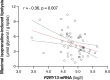
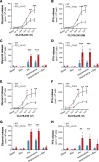
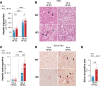
Excessive lipolysis in white adipose tissue (WAT) leads to insulin resistance (IR) and ectopic fat accumulation in insulin-sensitive tissues. However, the impact of Gi-coupled receptors in restraining adipocyte lipolysis through inhibition of cAMP production remained poorly elucidated. Given that the Gi-coupled P2Y13 receptor (P2Y13-R) is a purinergic receptor expressed in WAT, we investigated its role in adipocyte lipolysis and its effect on IR and metabolic dysfunction-associated steatotic liver disease (MASLD). In humans, mRNA expression of P2Y13-R in WAT was negatively correlated to adipocyte lipolysis. In mice, adipocytes lacking P2Y13-R displayed higher intracellular cAMP levels, indicating impaired Gi signaling. Consistently, the absence of P2Y13-R was linked to increased lipolysis in adipocytes and WAT explants via hormone-sensitive lipase activation. Metabolic studies indicated that mice lacking P2Y13-R showed a greater susceptibility to diet-induced IR, systemic inflammation, and MASLD compared with their wild-type counterparts. Assays conducted on precision-cut liver slices exposed to WAT conditioned medium and on liver-specific P2Y13-R-knockdown mice suggested that P2Y13-R activity in WAT protects from hepatic steatosis, independently of liver P2Y13-R expression. In conclusion, our findings support the idea that targeting adipose P2Y13-R activity may represent a pharmacological strategy to prevent obesity-associated disorders, including type 2 diabetes and MASLD.
Keywords: Adipose tissue; G protein–coupled receptors; Glucose metabolism; Hepatology; Metabolism.
Figures




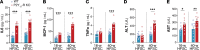


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

