Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications
- PMID: 38470772
- PMCID: PMC10934113
- DOI: 10.3390/nano14050441
Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications
Abstract
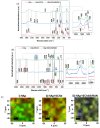
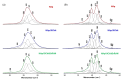
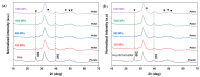
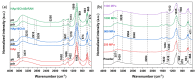
In the biomedical field, nanocrystalline hydroxyapatite is still one of the most attractive candidates as a bone substitute material due to its analogies with native bone mineral features regarding chemical composition, bioactivity and osteoconductivity. Ion substitution and low crystallinity are also fundamental characteristics of bone apatite, making it metastable, bioresorbable and reactive. In the present work, biomimetic apatite and apatite/chitosan composites were produced by dissolution-precipitation synthesis, using mussel shells as a calcium biogenic source. With an eye on possible bone reconstruction and drug delivery applications, apatite/chitosan composites were loaded with strontium ranelate, an antiosteoporotic drug. Due to the metastability and temperature sensitivity of the produced composites, sintering could be carried out by conventional methods, and therefore, cold sintering was selected for the densification of the materials. The composites were consolidated up to ~90% relative density by applying a uniaxial pressure up to 1.5 GPa at room temperature for 10 min. Both the synthesised powders and cold-sintered samples were characterised from a physical and chemical point of view to demonstrate the effective production of biomimetic apatite/chitosan composites from mussel shells and exclude possible structural changes after sintering. Preliminary in vitro tests were also performed, which revealed a sustained release of strontium ranelate for about 19 days and no cytotoxicity towards human osteoblastic-like cells (MG63) exposed up to 72 h to the drug-containing composite extract.
Keywords: apatite/chitosan composites; chitosan; cold sintering; dissolution–precipitation synthesis; drug delivery; mussel shells; nanocrystalline apatite; strontium ranelate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Wang G.C., Lu Z.F., Zreiqat H. Bone Substitute Biomaterials. Elsevier; Amsterdam, The Netherlands: 2014. Bioceramics for skeletal bone regeneration; pp. 180–216.
-
- Dorozhkin S.V. Current State of Bioceramics. J. Ceram. Sci. Technol. 2018;9:353–370. doi: 10.4416/JCST2018-00026. - DOI
-
- Fiume E., Magnaterra G., Rahdar A., Verné E., Baino F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics. 2021;4:542–563. doi: 10.3390/ceramics4040039. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

