Tailoring Mesoporous Silica-Coated Silver Nanoparticles and Polyurethane-Doped Films for Enhanced Antimicrobial Applications
- PMID: 38470791
- PMCID: PMC10934637
- DOI: 10.3390/nano14050462
Tailoring Mesoporous Silica-Coated Silver Nanoparticles and Polyurethane-Doped Films for Enhanced Antimicrobial Applications
Abstract
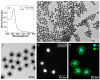
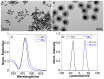
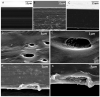
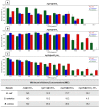
The global increase in multidrug-resistant bacteria poses a challenge to public health and requires the development of new antibacterial materials. In this study, we examined the bactericidal properties of mesoporous silica-coated silver nanoparticles, varying the core sizes (ca. 28 nm and 51 nm). We also investigated gold nanoparticles (ca. 26 nm) coated with mesoporous silica as possible inert metal cores. To investigate the modification of antimicrobial activity after the surface charge change, we used silver nanoparticles with a silver core of 28 nm coated with a mesoporous shell (ca. 16 nm) and functionalized with a terminal amine group. Furthermore, we developed a facile method to create mesoporous silica-coated silver nanoparticles (Ag@mSiO2) doped films using polyurethane (IROGRAN®) as a polymer matrix via solution casting. The antibacterial effects of silver nanoparticles with different core sizes were analyzed against Gram-negative and Gram-positive bacteria relevant to the healthcare and food industry. The results demonstrated that gold nanoparticles were inert, while silver nanoparticles exhibited antibacterial effects against Gram-negative (Escherichia coli and Salmonella enterica subsp. enterica serovar Choleraesuis) and Gram-positive (Bacillus cereus) strains. In particular, the larger Ag@mSiO2 nanoparticles showed a minimum inhibitory concentration (MIC) and a minimum bactericidal concentration (MBC) of 18 µg/mL in the Salmonella strain. Furthermore, upon terminal amine functionalization, reversing the surface charge to positive values, there was a significant increase in the antibacterial activity of the NPs compared to their negative counterparts. Finally, the antimicrobial properties of the nanoparticle-doped polyurethane films revealed a substantial improvement in antibacterial efficacy. This study provides valuable information on the potential of mesoporous silica-coated silver nanoparticles and their applications in fighting multidrug-resistant bacteria, especially in the healthcare and food industries.
Keywords: antimicrobial activity; bacterials; gold nanoparticles; polymers; silica core shell; silver nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

