G-CSF + plerixafor versus G-CSF alone mobilized hematopoietic stem cells in patients with multiple myeloma and lymphoma: a systematic review and meta-analysis
- PMID: 38470973
- PMCID: PMC10939106
- DOI: 10.1080/07853890.2024.2329140
G-CSF + plerixafor versus G-CSF alone mobilized hematopoietic stem cells in patients with multiple myeloma and lymphoma: a systematic review and meta-analysis
Abstract
Aim: The combination of granulocyte-colony stimulating factor (G-CSF) and plerixafor is one of the approaches for hematopoietic stem cell mobilization in patients with multiple myeloma (MM), non-Hodgkin's lymphoma (NHL), and Hodgkin's lymphoma (HL). This systematic review and meta-analysis aimed to determine the ability of G-CSF + plerixafor to mobilize peripheral blood (PB) CD34+ cells and examine its safety profile.
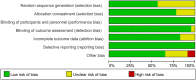
Methods: We performed a database search using the terms 'granulocyte colony stimulating factor', 'G-CSF', 'AMD3100', and 'plerixafor', published up to May 1, 2023. The methodology is described in further detail in the PROSPERO database (CRD42023425760).
Results: Twenty-three studies were included in this systematic review and meta-analysis. G-CSF + plerixafor resulted in more patients achieving the predetermined apheresis yield of CD34+ cells than G-CSF alone (OR, 5.33; 95%, 4.34-6.55). It was further discovered that G-CSF + plerixafor could mobilize more CD34+ cells into PB, which was beneficial for the next transplantation in both randomized controlled (MD, 18.30; 95%, 8.74-27.85) and single-arm (MD, 20.67; 95%, 14.34-27.00) trials. Furthermore, G-CSF + plerixafor did not cause more treatment emergent adverse events than G-CSF alone (OR, 1.25; 95%, 0.87-1.80).
Conclusions: This study suggests that the combination of G-CSF and plerixafor, resulted in more patients with MM, NHL, and HL, achieving the predetermined apheresis yield of CD34+ cells, which is related to the more effective mobilization of CD34+ cells into PB.
Keywords: CD34+ cell; First-line; G-CSF; HL; MM; NHL; Plerixafor.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: results of the prospective PREDICT trial.Haematologica. 2013 Feb;98(2):172-8. doi: 10.3324/haematol.2012.071456. Epub 2012 Sep 14. Haematologica. 2013. PMID: 22983579 Free PMC article. Clinical Trial.
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
-
Successful Mobilization of Autologous Hematopoietic Peripheral Blood Stem Cells after Salvage Chemotherapy in Patients with Low CD34 Blood Cell Counts.Transplant Cell Ther. 2022 Nov;28(11):754-759. doi: 10.1016/j.jtct.2022.08.017. Epub 2022 Aug 22. Transplant Cell Ther. 2022. PMID: 36002104
-
[Efficiency and safety analysis of Plerixafor combined with granulocyte colony-stimulating factor on autologous hematopoietic stem cell mobilization in lymphoma].Zhonghua Xue Ye Xue Za Zhi. 2023 Feb 14;44(2):112-117. doi: 10.3760/cma.j.issn.0253-2727.2023.02.005. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 36948864 Free PMC article. Chinese.
-
Plerixafor for stem cell mobilization in patients with non-Hodgkin's lymphoma and multiple myeloma.Ann Pharmacother. 2010 Jan;44(1):117-26. doi: 10.1345/aph.1M380. Epub 2009 Dec 15. Ann Pharmacother. 2010. PMID: 20009003 Review.
Cited by
-
Comparing stem cell mobilization with chemotherapy and cytokine (G-CSF) versus cytokine alone in myeloma patients (MOCCCA): a randomized phase II, open-label, non-inferiority trial.Bone Marrow Transplant. 2025 Mar;60(3):270-276. doi: 10.1038/s41409-024-02468-z. Epub 2024 Nov 15. Bone Marrow Transplant. 2025. PMID: 39548306 Free PMC article. Clinical Trial.
References
-
- Dimopoulos MA, Richardson PG, Bahlis NJ, et al. . Addition of elotuzumab to lenalidomide and dexamethasone for patients with newly diagnosed, transplantation ineligible multiple myeloma (ELOQUENT-1): an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022;9(6):e403–e414. doi:10.1016/S2352-3026(22)00103-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
