G-CSF + plerixafor versus G-CSF alone mobilized hematopoietic stem cells in patients with multiple myeloma and lymphoma: a systematic review and meta-analysis
- PMID: 38470973
- PMCID: PMC10939106
- DOI: 10.1080/07853890.2024.2329140
G-CSF + plerixafor versus G-CSF alone mobilized hematopoietic stem cells in patients with multiple myeloma and lymphoma: a systematic review and meta-analysis
Abstract
Aim: The combination of granulocyte-colony stimulating factor (G-CSF) and plerixafor is one of the approaches for hematopoietic stem cell mobilization in patients with multiple myeloma (MM), non-Hodgkin's lymphoma (NHL), and Hodgkin's lymphoma (HL). This systematic review and meta-analysis aimed to determine the ability of G-CSF + plerixafor to mobilize peripheral blood (PB) CD34+ cells and examine its safety profile.
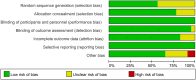
Methods: We performed a database search using the terms 'granulocyte colony stimulating factor', 'G-CSF', 'AMD3100', and 'plerixafor', published up to May 1, 2023. The methodology is described in further detail in the PROSPERO database (CRD42023425760).
Results: Twenty-three studies were included in this systematic review and meta-analysis. G-CSF + plerixafor resulted in more patients achieving the predetermined apheresis yield of CD34+ cells than G-CSF alone (OR, 5.33; 95%, 4.34-6.55). It was further discovered that G-CSF + plerixafor could mobilize more CD34+ cells into PB, which was beneficial for the next transplantation in both randomized controlled (MD, 18.30; 95%, 8.74-27.85) and single-arm (MD, 20.67; 95%, 14.34-27.00) trials. Furthermore, G-CSF + plerixafor did not cause more treatment emergent adverse events than G-CSF alone (OR, 1.25; 95%, 0.87-1.80).
Conclusions: This study suggests that the combination of G-CSF and plerixafor, resulted in more patients with MM, NHL, and HL, achieving the predetermined apheresis yield of CD34+ cells, which is related to the more effective mobilization of CD34+ cells into PB.
Keywords: CD34+ cell; First-line; G-CSF; HL; MM; NHL; Plerixafor.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Dimopoulos MA, Richardson PG, Bahlis NJ, et al. Addition of elotuzumab to lenalidomide and dexamethasone for patients with newly diagnosed, transplantation ineligible multiple myeloma (ELOQUENT-1): an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022;9(6):e403–e414. doi: 10.1016/S2352-3026(22)00103-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
