Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3
- PMID: 38471061
- PMCID: PMC11095884
- DOI: 10.1200/JCO.23.02474
Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3
Abstract
Purpose: Allogeneic hematopoietic cell transplantation (HCT) improves outcomes for patients with AML harboring an internal tandem duplication mutation of FLT3 (FLT3-ITD) AML. These patients are routinely treated with a FLT3 inhibitor after HCT, but there is limited evidence to support this. Accordingly, we conducted a randomized trial of post-HCT maintenance with the FLT3 inhibitor gilteritinib (ClinicalTrials.gov identifier: NCT02997202) to determine if all such patients benefit or if detection of measurable residual disease (MRD) could identify those who might benefit.
Methods: Adults with FLT3-ITD AML in first remission underwent HCT and were randomly assigned to placebo or 120 mg once daily gilteritinib for 24 months after HCT. The primary end point was relapse-free survival (RFS). Secondary end points included overall survival (OS) and the effect of MRD pre- and post-HCT on RFS and OS.
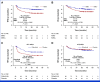
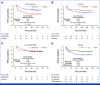
Results: Three hundred fifty-six participants were randomly assigned post-HCT to receive gilteritinib or placebo. Although RFS was higher in the gilteritinib arm, the difference was not statistically significant (hazard ratio [HR], 0.679 [95% CI, 0.459 to 1.005]; two-sided P = .0518). However, 50.5% of participants had MRD detectable pre- or post-HCT, and, in a prespecified subgroup analysis, gilteritinib was beneficial in this population (HR, 0.515 [95% CI, 0.316 to 0.838]; P = .0065). Those without detectable MRD showed no benefit (HR, 1.213 [95% CI, 0.616 to 2.387]; P = .575).
Conclusion: Although the overall improvement in RFS was not statistically significant, RFS was higher for participants with detectable FLT3-ITD MRD pre- or post-HCT who received gilteritinib treatment. To our knowledge, these data are among the first to support the effectiveness of MRD-based post-HCT therapy.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. - PubMed
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. - PubMed
-
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:721–749. - PubMed
-
- NCCN Guidelines: NCCN Clinical Practice Guidelines in Oncology for Guideline for AML 2023. http://NCCN.org
Publication types
MeSH terms
Associated data
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- UG1 HL069249/HL/NHLBI NIH HHS/United States
- UG1 HL138645/HL/NHLBI NIH HHS/United States
- UG1 HL108945/HL/NHLBI NIH HHS/United States
- UG1 HL108987/HL/NHLBI NIH HHS/United States
- UG1 HL069246/HL/NHLBI NIH HHS/United States
- R50 CA275927/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- UG1 HL069291/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
- UG1 HL069310/HL/NHLBI NIH HHS/United States
- U10 HL069301/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 HL109137/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

