Third-party fecal microbiota transplantation for high-risk treatment-naïve acute GVHD of the lower GI tract
- PMID: 38471063
- PMCID: PMC11063394
- DOI: 10.1182/bloodadvances.2024012556
Third-party fecal microbiota transplantation for high-risk treatment-naïve acute GVHD of the lower GI tract
Abstract
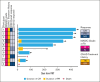
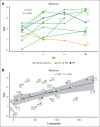
Disruption of the intestinal microbiome is observed with acute graft-versus-host disease (GVHD) of the lower gastrointestinal (LGI) tract, and fecal microbiota transplantation (FMT) has successfully cured steroid-refractory cases. In this open-label, single-arm, pilot study, third-party, single-donor FMT was administered in combination with systemic corticosteroids to participants with high-risk acute LGI GVHD, with a focus on treatment-naïve cases. Participants were scheduled to receive 1 induction dose (15 capsules per day for 2 consecutive days), followed by 3 weekly maintenance doses, consisting of 15 capsules per dose. The primary end point of the study was feasibility, which would be achieved if ≥80% of participants able to swallow ≥40 of the 75 scheduled capsules. Ten participants (9 treatment-naïve; 1 steroid-refractory) were enrolled and treated. The study met the primary end point, with 9 of 10 participants completing all eligible doses. Organ-specific LGI complete response rate at day 28 was 70%. Initial clinical response was observed within 1 week for all responders, and clinical responses were durable without recurrent LGI GVHD in complete responders. Exploratory analyses suggest that alpha diversity increased after FMT. Although recipient microbiome composition never achieved a high degree of donor similarity, expansion of donor-derived species and increases in tryptophan metabolites and short-chain fatty acids were observed within the first 7 days after FMT. Investigation into the use of microbiome-targeted interventions earlier in the treatment paradigm for acute LGI GVHD is warranted. This trial was registered at www.ClinicalTrials.gov as #NCT04139577.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: Z.D. receives research support from Incyte, REGiMMUNE, and Taiho Oncology; and has received consulting fees from Sanofi, Incyte, MorphoSys, Inhibrx, PharmaBiome, and ONO Pharmaceutical. R.A.N. has received equity from TimeDoc Health. M.J.F. receives research support from Incyte, Arcellx, Novartis, and Kite; and has received consulting fees from Kite, Novartis, Bristol Myers Squibb, and Iovance Biotherapeutics. T.R.S. has served on committees for bluebird bio (data monitoring committee), Syneos Health (data monitoring committee and adjudication committee), Ossium Health (scientific review committee); and has served on a scientific advisory board for Qihan Biotech. R.R.J. is an adviser and holds equity in Seres Therapeutics and Kaleido Biosciences; serves on the advisory board of MaaT Pharma, LISCure Biosciences, and Prolacta Biosciences; and consults for Da Volterra, Merck, Microbiome DX, and Karius. Y.-B.C. has received consulting fees from Takeda, Incyte, Vor Bio, Pharmacosmos, Editas, and Celularity. The remaining authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

