A Histone Methylation-MAPK Signaling Axis Drives Durable Epithelial-Mesenchymal Transition in Hypoxic Pancreatic Cancer
- PMID: 38471099
- PMCID: PMC12032584
- DOI: 10.1158/0008-5472.CAN-22-2945
A Histone Methylation-MAPK Signaling Axis Drives Durable Epithelial-Mesenchymal Transition in Hypoxic Pancreatic Cancer
Abstract
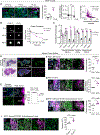
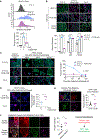
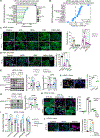
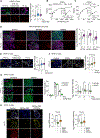
The tumor microenvironment in pancreatic ductal adenocarcinoma (PDAC) plays a key role in tumor progression and response to therapy. The dense PDAC stroma causes hypovascularity, which leads to hypoxia. Here, we showed that hypoxia drives long-lasting epithelial-mesenchymal transition (EMT) in PDAC primarily through a positive-feedback histone methylation-MAPK signaling axis. Transformed cells preferentially underwent EMT in hypoxic tumor regions in multiple model systems. Hypoxia drove a cell autonomous EMT in PDAC cells, which, unlike EMT in response to growth factors, could last for weeks. Furthermore, hypoxia reduced histone demethylase KDM2A activity, suppressed PP2 family phosphatase expression, and activated MAPKs to post-translationally stabilize histone methyltransferase NSD2, leading to an H3K36me2-dependent EMT in which hypoxia-inducible factors played only a supporting role. Hypoxia-driven EMT could be antagonized in vivo by combinations of MAPK inhibitors. Collectively, these results suggest that hypoxia promotes durable EMT in PDAC by inducing a histone methylation-MAPK axis that can be effectively targeted with multidrug therapies, providing a potential strategy for overcoming chemoresistance.
Significance: Integrated regulation of histone methylation and MAPK signaling by the low-oxygen environment of pancreatic cancer drives long-lasting EMT that promotes chemoresistance and shortens patient survival and that can be pharmacologically inhibited. See related commentary by Wirth and Schneider, p. 1739.
©2024 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest: The authors declare no conflicts of interest.
Figures



References
-
- Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000;48:919–22 - PubMed
-
- Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res 2011;71:3110–20 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

