Reproductive Ageing: Current insights and a potential role of NAD in the reproductive health of aging fathers and their children
- PMID: 38471307
- PMCID: PMC11075800
- DOI: 10.1530/REP-23-0486
Reproductive Ageing: Current insights and a potential role of NAD in the reproductive health of aging fathers and their children
Abstract
In brief: In light of the increasing age of first-time fathers, this article summarizes the current scientific knowledge base on reproductive aging in the male, including sperm quality and health impacts for the offspring. The emerging role of NAD decline in reproductive aging is highlighted.
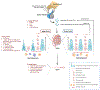
Abstract: Over the past decades, the age of first-time fathers has been steadily increasing due to socio-economic pressures. While general mechanisms of aging are subject to intensive research, male reproductive aging has remained an understudied area, and the effects of increased age on the male reproductive system are still only poorly understood, despite new insights into the potential dire consequences of advanced paternal age for the health of their progeny. There is also growing evidence that reproductive aging is linked to overall health in men, but this review mainly focuses on pathophysiological consequences of old age in men, such as low sperm count and diminished sperm genetic integrity, with an emphasis on mechanisms underlying reproductive aging. The steady decline of NAD levels observed in aging men represents one of the emerging concepts in that regard. Because it offers some mechanistic rationale explaining the effects of old age on the male reproductive system, some of the NAD-dependent functions in male reproduction are briefly outlined in this review. The overview also provides many questions that remain open about the basic science of male reproductive aging.
Conflict of interest statement
Declaration in interest
The authors declare no conflicts of interest.
Figures


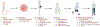
Similar articles
-
Paternal age negatively affects sperm production of the progeny.Ecol Lett. 2021 Apr;24(4):719-727. doi: 10.1111/ele.13696. Epub 2021 Feb 10. Ecol Lett. 2021. PMID: 33565248
-
Advanced paternal age: effects on sperm parameters, assisted reproduction outcomes and offspring health.Reprod Biol Endocrinol. 2020 Nov 13;18(1):110. doi: 10.1186/s12958-020-00668-y. Reprod Biol Endocrinol. 2020. PMID: 33183337 Free PMC article. Review.
-
Age-associated epigenetic changes in mammalian sperm: implications for offspring health and development.Hum Reprod Update. 2023 Jan 5;29(1):24-44. doi: 10.1093/humupd/dmac033. Hum Reprod Update. 2023. PMID: 36066418 Free PMC article. Review.
-
The high-priority ethical issues of advanced paternal age: perspectives from a panel of experts in the fields of men reproduction and family building.BMC Med Ethics. 2025 Apr 12;26(1):46. doi: 10.1186/s12910-025-01202-8. BMC Med Ethics. 2025. PMID: 40217476 Free PMC article.
-
Relationship between sperm NAD + concentration and reproductive aging in normozoospermia men:A Cohort study.BMC Urol. 2022 Oct 1;22(1):159. doi: 10.1186/s12894-022-01107-3. BMC Urol. 2022. PMID: 36182928 Free PMC article.
Cited by
-
Research Progress on the Interaction Between SIRT1 and Mitochondrial Biochemistry in the Aging of the Reproductive System.Biology (Basel). 2025 Jun 2;14(6):643. doi: 10.3390/biology14060643. Biology (Basel). 2025. PMID: 40563894 Free PMC article. Review.
-
Autophagy and Female Fertility: Mechanisms, Clinical Implications, and Emerging Therapies.Cells. 2024 Aug 14;13(16):1354. doi: 10.3390/cells13161354. Cells. 2024. PMID: 39195244 Free PMC article. Review.
-
Reproductive toxicology: keeping up with our changing world.Front Toxicol. 2024 Oct 11;6:1456687. doi: 10.3389/ftox.2024.1456687. eCollection 2024. Front Toxicol. 2024. PMID: 39463893 Free PMC article.
References
-
- Aitken RJ (2022) Role of sperm DNA damage in creating de-novo mutations in human offspring: the ‘post-meiotic oocyte collusion’ hypothesis. Reproductive BioMedicine Online 45 109–124. - PubMed
-
- Aitken RJ and Bakos HW (2021) Should we be measuring DNA damage in human spermatozoa? New light on an old question. Human Reproduction 36 1175–1185. - PubMed
-
- Aitken RJ and Lewis SEM (2023) DNA damage in testicular germ cells and spermatozoa. When and how is it induced? How should we measure it? What does it mean? Andrology 11 1545–1557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

