Synthetic PET from CT improves diagnosis and prognosis for lung cancer: Proof of concept
- PMID: 38471502
- PMCID: PMC10983039
- DOI: 10.1016/j.xcrm.2024.101463
Synthetic PET from CT improves diagnosis and prognosis for lung cancer: Proof of concept
Abstract
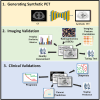
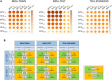
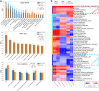
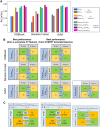
[18F]Fluorodeoxyglucose positron emission tomography (FDG-PET) and computed tomography (CT) are indispensable components in modern medicine. Although PET can provide additional diagnostic value, it is costly and not universally accessible, particularly in low-income countries. To bridge this gap, we have developed a conditional generative adversarial network pipeline that can produce FDG-PET from diagnostic CT scans based on multi-center multi-modal lung cancer datasets (n = 1,478). Synthetic PET images are validated across imaging, biological, and clinical aspects. Radiologists confirm comparable imaging quality and tumor contrast between synthetic and actual PET scans. Radiogenomics analysis further proves that the dysregulated cancer hallmark pathways of synthetic PET are consistent with actual PET. We also demonstrate the clinical values of synthetic PET in improving lung cancer diagnosis, staging, risk prediction, and prognosis. Taken together, this proof-of-concept study testifies to the feasibility of applying deep learning to obtain high-fidelity PET translated from CT.
Keywords: Turing test; generative AI; generative adversarial network; image synthesis; lung cancer; prognostic marker; radiogenomics; radiomics; screening; synthetic PET.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.C. reports speaker fees and honoraria from The Society for Immunotherapy of Cancer, Bristol Myers Squibb, Roche, Medscape, and PeerView; having an advisory role or receiving consulting fees from AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck & Co., Genentech, and Arrowhead Pharmaceuticals; and institutional research funding from AstraZeneca, Bristol Myers Squibb, and EMD Serono. L.Y. has grant support from Lantheus Inc. D.L.G. has served on scientific advisory committees for Menarini Ricerche, 4D Pharma, Onconova, and Eli Lilly and has received research support from Takeda, Astellas, NGM Biopharmaceuticals, Boehringer Ingelheim, and AstraZeneca. N.I.V. receives consulting fees from Sanofi, Regeneron, Oncocyte, and Eli Lilly and research funding from Mirati, outside the submitted work. J.D.H. is on the Scientific Advisory Board of Imagion Biosystems. J.Y.C. reports research funding from BMS-MDACC and Siemens Healthcare, and consultation fees from Legion Healthcare Partners. L.Y. has grant support from Lantheus Inc. M.C.B.G. has received research funding from Siemens Healthcare. I.W. has received honoraria from Genentech/Roche, AstraZeneca, Merck, Guardant Health, Flame, Novartis, Sanofi, Daiichi Sankyo, Dava Oncology, Amgen, GlaxoSmithKline, HTG Molecular, Jansen, Merus, Imagene, G1 Therapeutics, Abbvie, Catalyst Therapeutics, Genzyme, Regeneron, Oncocyte, Medscape, Platform Health, Pfizer, Physicians’ Education Resource, HPM Education, and Aptitute Health; additionally, I.W. has received research support from Genentech, Merck, Bristol-Myers Squibb, Medimmune, Adaptive, Adaptimmune, EMD Serono, Pfizer, Takeda, Amgen, Karus, Johnson & Johnson, Bayer, Iovance, 4D, Novartis, and Akoya. D.L.G. has served on scientific advisory committees for Menarini Ricerche, 4D Pharma, Onconova, and Eli Lilly and has received research support from Takeda, Astellas, NGM Biopharmaceuticals, Boehringer Ingelheim, and AstraZeneca. J.V.H. reports being on scientific advisory boards for AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Eli Lilly, Novartis, Spectrum, EMD Serono, Sanofi, Takeda, Mirati Therapeutics, BMS, and Janssen Global Services; receiving research support from AstraZeneca, Takeda, Boehringer Ingelheim, and Spectrum; and receiving licensing fees from Spectrum. C.C.W. reports research support from Medical Imaging and Data Resource Center from NIBIB/University of Chicago and royalties from Elsevier. J.Z. reports serving on the consulting/advisory board of Bristol-Myers Squibb, AstraZeneca, Novartis, Johnson & Johnson, GenePlus, Innovent, Varian, and Catalyst, and receiving research grants to institution from Merck, Novartis, and Johnson & Johnson. J.W. reports research funding from Siemens Healthcare.
Figures


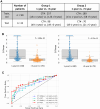
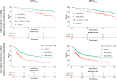
References
-
- Fletcher J.W., Djulbegovic B., Soares H.P., Siegel B.A., Lowe V.J., Lyman G.H., Coleman R.E., Wahl R., Paschold J.C., Avril N., et al. Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med. 2008;49:480–508. - PubMed
-
- Garcia-Velloso M.J., Bastarrika G., de-Torres J.P., Lozano M.D., Sanchez-Salcedo P., Sancho L., Nuñez-Cordoba J.M., Campo A., Alcaide A.B., Torre W., et al. Assessment of indeterminate pulmonary nodules detected in lung cancer screening: Diagnostic accuracy of FDG PET/CT. Lung Cancer. 2016;97:81–86. - PubMed
-
- Shim S.S., Lee K.S., Kim B.-T., Chung M.J., Lee E.J., Han J., Choi J.Y., Kwon O.J., Shim Y.M., Kim S. Non–small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. - PubMed
-
- Gallach M., Mikhail Lette M., Abdel-Wahab M., Giammarile F., Pellet O., Paez D. Addressing global inequities in positron emission tomography-computed tomography (PET-CT) for cancer management: a statistical model to guide strategic planning. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 e926544-e926541. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

