Protocol for the development of a tool (INSPECT-SR) to identify problematic randomised controlled trials in systematic reviews of health interventions
- PMID: 38471680
- PMCID: PMC10936473
- DOI: 10.1136/bmjopen-2024-084164
Protocol for the development of a tool (INSPECT-SR) to identify problematic randomised controlled trials in systematic reviews of health interventions
Abstract
Introduction: Randomised controlled trials (RCTs) inform healthcare decisions. It is now apparent that some published RCTs contain false data and some appear to have been entirely fabricated. Systematic reviews are performed to identify and synthesise all RCTs that have been conducted on a given topic. While it is usual to assess methodological features of the RCTs in the process of undertaking a systematic review, it is not usual to consider whether the RCTs contain false data. Studies containing false data therefore go unnoticed and contribute to systematic review conclusions. The INveStigating ProblEmatic Clinical Trials in Systematic Reviews (INSPECT-SR) project will develop a tool to assess the trustworthiness of RCTs in systematic reviews of healthcare-related interventions.
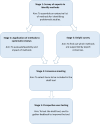
Methods and analysis: The INSPECT-SR tool will be developed using expert consensus in combination with empirical evidence, over five stages: (1) a survey of experts to assemble a comprehensive list of checks for detecting problematic RCTs, (2) an evaluation of the feasibility and impact of applying the checks to systematic reviews, (3) a Delphi survey to determine which of the checks are supported by expert consensus, culminating in, (4) a consensus meeting to select checks to be included in a draft tool and to determine its format and (5) prospective testing of the draft tool in the production of new health systematic reviews, to allow refinement based on user feedback. We anticipate that the INSPECT-SR tool will help researchers to identify problematic studies and will help patients by protecting them from the influence of false data on their healthcare.
Ethics and dissemination: The University of Manchester ethics decision tool was used, and this returned the result that ethical approval was not required for this project (30 September 2022), which incorporates secondary research and surveys of professionals about subjects relating to their expertise. Informed consent will be obtained from all survey participants. All results will be published as open-access articles. The final tool will be made freely available.
Keywords: Randomized Controlled Trial; STATISTICS & RESEARCH METHODS; Systematic Review.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JW, CH, GAA, LB and JJK declare funding from NIHR (NIHR203568) in relation to this work. JW additionally declares stats or methodological editor roles for BJOG, Fertility and Sterility, Reproduction and Fertility, Journal of Hypertension and for Cochrane Gynaecology and Fertility. CH declares a statistical editor role for Cochrane Colorectal. LB additionally declares a role as academic meta-research editor for PLoS Biology, and that The University of Colorado receives remuneration for service as senior research integrity editor, Cochrane. JJK additionally declares a statistical editor role for The BMJ. WL, LS and RW declare funding from Australian National Health and Medical Research Council Investigator Grants (GNT2016729, GNT2009432, GNT2009767). LS additionally declares roles as editorial board member for Cochrane Evidence Synthesis Methods and statistical editor for Cochrane Neonatal Group. RW additionally declares roles as deputy editor for Human Reproduction and editorial board member for BJOG and Cochrane Gynaecology and Fertility. WL additionally declares roles of associate editor for Human Reproduction Open and methodological editor for Fertility and Sterility and Fertility and Sterility Reviews. EF, SG and TLasserson declare employment by Cochrane. EF additionally declares a role as editorial board member for Cochrane Synthesis and Methods. TLasserson additionally declares authorship of a chapter in the Cochrane Handbook for Systematic Reviews of Interventions and that he is a developer of standards for Cochrane Intervention Reviews (MECIR). SG additionally declares an academic editor role for PLOS Global Public Health. ES declares that she was a member of the Cochrane scientific misconduct policy advisory group, and roles as sign-off editor and proposal editor of The Cochrane Library. ZA declares he is a member of the Cochrane Library Editorial Board, was co-ordinating editor for Cochrane Pregnancy and Childbirth Group 2002–2023 and is PI on a grant from Children Investment Foundation Fund to University of Liverpool to investigate research integrity of clinical trials related to nutritional supplements in pregnancy. TLi is supported by grant UG1 EY020522 from the National Eye Institute, National Institutes of Health. TLi also declares roles as editor-in-chief for Trials, statistical editor for Annals of Internal Medicine, review editor for JAMA Ophthalmology and editorial board member for Journal of Clinical Epidemiology. MC declares that he is Co-ordinating editor for the Cochrane Methodology Review Group, Editor in Chief of Journal of Evidence-Based Medicine, and Co-ordinating Editor of the James Lind Library. AA declares that The Health Services Research Unit, University of Aberdeen, is funded by the Health and Social Care Directorates of the Scottish Government. All other authors declare no competing interests. DT declares an RCT topic editor role for Research Methods in Medicine and Health Sciences. MvW declares roles as co-ordinating editor for Cochrane Gynaecology and Fertility and Sexually Transmitted Infections, methodology editor for Human Reproduction Update and editorial editor for Fertility and Sterility. NEO declares roles as co-ordinating editor for Cochrane Pain, Palliative and Supportive Care (PaPaS) Group, 2020–2023, as member of Cochrane Central Editorial Board and as editorial board member for Journal of Pain. JC declares an editor role for Anaesthesia. AL declares a role as editorial board member for BMC Medical Ethics. GAA declares a role as statistical editor for the European Journal of Vascular and Endovascular Surgery. NJLB declares roles as editorial board member for International Review of Social Psychology/Revue Internationale de Psychologie Sociale, statistical advisory board member for Mental Health Science and advisory board member for Meta-Psychology. BWM declares roles as editor for Cochrane Gynaecology and Fertility and Sexually Transmitted Infections and for Fertility and Sterility. SL declares roles as associate editor for Human Reproduction, methodological editor for Fertility and Sterility and editor for Cochrane Gynaecology and Fertility. EL is head of research and clinical editor for The BMJ.
Figures
Update of
-
Protocol for the development of a tool (INSPECT-SR) to identify problematic randomised controlled trials in systematic reviews of health interventions.medRxiv [Preprint]. 2023 Nov 13:2023.09.21.23295626. doi: 10.1101/2023.09.21.23295626. medRxiv. 2023. Update in: BMJ Open. 2024 Mar 11;14(3):e084164. doi: 10.1136/bmjopen-2024-084164. PMID: 37873409 Free PMC article. Updated. Preprint.
References
-
- Brown NJL. 2023. Available: http://steamtraen.blogspot.com/2021/07/Some-problems-with-the-data-from-...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials