Association between expedited review designations and the US or global burden of disease for drugs approved by the US Food and Drug Administration, 2010-2019: a cross-sectional analysis
- PMID: 38471684
- PMCID: PMC10936494
- DOI: 10.1136/bmjopen-2023-076542
Association between expedited review designations and the US or global burden of disease for drugs approved by the US Food and Drug Administration, 2010-2019: a cross-sectional analysis
Abstract
Objectives: Pharmaceutical innovation can contribute to reducing the burden of disease in human populations. This research asks whether products approved by the US Food and Drug Administration (FDA) from 2010 to 2019 and expedited review programmes incentivising development of products for serious disease were aligned with the US or global burden of disease.
Design: Cross-sectional study.
Outcome measures: Association of FDA product approvals (2010-2019), first approved indications, designations for expedited review with the burden of disease (disability-adjusted life years (DALYs)), years of life lost (YLL) and years of life lived with disability (YLD) for 122 WHO Global Health Estimates (GHE) conditions in US and global (ex-US) populations.
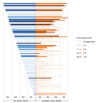
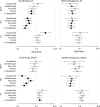
Results: The FDA approved 387 drugs in 2010-2019 with lead indications associated with 59/122 GHE conditions. Conditions with at least one new drug had greater US DALYs (p=0.001), US YLL (p<0.001), global DALYs (p=0.030) and global YLL (p=0.004) but not US YLD (p=0.158) or global YLD (p=0.676). Most approvals were for conditions in the top quartile of US DALYs or YLL, but <27% were for conditions in the top quartile of global DALYs or YLL. The likelihood of a drug having one or more designations for expedited review programmes was negatively associated (OR<1) with US DALYs, US YLD and global YLD. There was a weak negative association with global DALYs and a weak positive association (OR>1) with US and global YLL.
Conclusions: FDA drug approvals from 2010 to 2019 were more strongly aligned with US than global disease burden. Designations for expedited review were not aligned with either the US or global burdens of disease and may inadvertently disincentivise development of products addressing global disease burdens. These results may inform policies to better align pharmaceutical innovation with the burdens of disease.
Keywords: Health policy; PUBLIC HEALTH; Quality of Life.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors report no competing interests. Since 2020, FL has received grant funding at Bentley University from the National Biomedical Research Foundation, Institute for New Economic Thinking, West Health Policy Center and the National Pharmaceutical Council.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
