Determination of Postmortem Interval in Mice
- PMID: 38471755
- PMCID: PMC11270044
- DOI: 10.30802/AALAS-JAALAS-23-000107
Determination of Postmortem Interval in Mice
Abstract
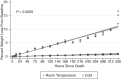
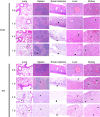
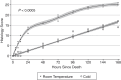
Despite the major use of mice in biomedical research, little information is available with regard to identifying their postmortem changes and using that information to determine the postmortem interval (PMI), defined as the time after death. Both PMI and environmental conditions influence decomposition (autolysis and putrefaction) and other postmortem changes. Severe decomposition compromises lesion interpretation and disease detection and wastes limited pathology resources. The goal of this study was to assess postmortem changes in mice in room temperature cage conditions and under refrigeration at 4 °C to develop gross criteria for the potential value of further gross and histologic evaluation. We used 108 experimentally naïve C57BL/6 mice that were humanely euthanized and then allocated them into 2 experimental groups for evaluation of postmortem change: room temperature (20 to 22 °C) or refrigeration (4 °C). PMI assessments, including gross changes and histologic scoring, were performed at hours 0, 4, 8, and 12 and on days 1 to 14. Factors such as temperature, humidity, ammonia in the cage, and weight change were also documented. Our data indicates that carcasses held at room temperature decomposed faster than refrigerated carcasses. For most tissues, decomposition was evident by 12 h at room temperature as compared with 5 d under refrigeration. At room temperature, gross changes were present by day 2 as compared with day 7 under refrigeration. Mice at room temperature lost 0.78% of their baseline body weight per day as compared with 0.06% for refrigerated mice (95% CI for difference 0.67% to 0.76%, P < 0.0005). This study supports the consideration of temperature and PMI as important factors affecting the suitability of postmortem tissues for gross and histologic evaluation and indicates that storage of carcasses under refrigeration will significantly slow autolysis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Almulhim AM, Menezes RG. [Internet]. 2023Evaluation of postmortem changes. In: StatPearls. Treasure Island (FL)StatPearls Publishing. [Cited 1 May 2023]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554464 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

