Sustained Pericarditis Recurrence Risk Reduction With Long-Term Rilonacept
- PMID: 38471825
- PMCID: PMC11010039
- DOI: 10.1161/JAHA.123.032516
Sustained Pericarditis Recurrence Risk Reduction With Long-Term Rilonacept
Abstract
Background: Rilonacept, a once-weekly interleukin-1 alpha and beta cytokine trap, reduced pericarditis recurrence in the phase 3 study, RHAPSODY (Rilonacept Inhibition of Interleukin-1 Alpha and Beta for Recurrent Pericarditis: A Pivotal Symptomatology and Outcomes Study). The RHAPSODY long-term extension further explored recurrent pericarditis natural history and treatment duration decision-making during 24 additional months of open-label rilonacept treatment.
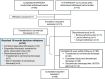
Methods and results: Seventy-four patients commenced the long-term extension, with a median (maximum) total rilonacept duration of 22 (35) months. Individually, 18 months after the most proximal pericarditis recurrence, investigators decided to continue rilonacept on study, suspend rilonacept for off-treatment observation (rescue allowed), or discontinue the study. The annualized incidence of pericarditis recurrence on rilonacept up to the 18-month decision milestone was 0.04 events/patient-year versus 4.4 events/patient-year prestudy while on oral therapies. At the 18-month decision milestone, 64% (33/52) continued rilonacept, 15% (8/52) suspended rilonacept for observation, and 21% (11/52) discontinued the study. Among the 33 patients (1/33; 3.0%) continuing rilonacept (median time to recurrence could not be estimated due to too few events), a single recurrence occurred 4 weeks after a treatment interruption. Among patients suspending rilonacept, 75% (6/8) experienced recurrence (median time to recurrence, 11.8 weeks [95% CI, 3.7 weeks to not estimable]). There was a 98% reduction in risk of pericarditis recurrence among patients continuing rilonacept treatment after the 18-month decision milestone versus those suspending treatment for observation (hazard ratio, 0.02; P<0.0001).
Conclusions: In the RHAPSODY long-term extension, continued rilonacept treatment resulted in continued response; treatment suspension at the 18-month decision milestone was associated with pericarditis recurrence.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03737110.
Keywords: autoinflammatory disease; interleukin‐1; recurrent pericarditis; rilonacept.
Figures