Hypoxia and low-glucose environments co-induced HGDILnc1 promote glycolysis and angiogenesis
- PMID: 38472215
- PMCID: PMC10933424
- DOI: 10.1038/s41420-024-01903-w
Hypoxia and low-glucose environments co-induced HGDILnc1 promote glycolysis and angiogenesis
Abstract
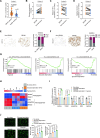
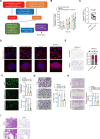
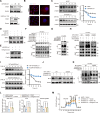
Small bowel vascular malformation disease (SBVM) commonly causes obscure gastrointestinal bleeding (OGIB). However, the pathogenetic mechanism and the role of lncRNAs in SBVM remain largely unknown. Here, we found that hypoxia and low-glucose environments co-augment angiogenesis and existed in SBVM. Mechanistically, hypoxia and low-glucose environments supported angiogenesis via activation of hypoxia and glucose deprivation-induced lncRNA (HGDILnc1) transcription by increasing binding of the NeuroD1 transcription factor to the HGDILnc1 promoter. Raised HGDILnc1 acted as a suppressor of α-Enolase 1 (ENO1) small ubiquitin-like modifier modification (SUMOylation)-triggered ubiquitination, and an activator of transcription of Aldolase C (ALDOC) via upregulation of Histone H2B lysine 16 acetylation (H2BK16ac) level in the promoter of ALDOC, and consequently promoting glycolysis and angiogenesis. Moreover, HGDILnc1 was clinically positively correlated with Neurogenic differentiation 1 (NeuroD1), ENO1, and ALDOC in SBVM tissues, and could function as a biomarker for SBVM diagnosis and therapy. These findings suggest that hypoxia and low-glucose environments were present in SBVM tissues, and co-augmented angiogenesis. Hypoxia and low-glucose environments co-induced HGDILnc1, which is higher expressed in SBVM tissue compared with normal tissue, could promoted glycolysis and angiogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Thalidomide targets EGFL6 to inhibit EGFL6/PAX6 axis-driven angiogenesis in small bowel vascular malformation.Cell Mol Life Sci. 2020 Dec;77(24):5207-5221. doi: 10.1007/s00018-020-03465-3. Epub 2020 Feb 1. Cell Mol Life Sci. 2020. PMID: 32008086 Free PMC article.
-
F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer.Gut. 2021 Nov;70(11):2123-2137. doi: 10.1136/gutjnl-2020-322780. Epub 2020 Dec 14. Gut. 2021. PMID: 33318144
-
MUC16 C-terminal binding with ALDOC disrupts the ability of ALDOC to sense glucose and promotes gallbladder carcinoma growth.Exp Cell Res. 2020 Sep 1;394(1):112118. doi: 10.1016/j.yexcr.2020.112118. Epub 2020 Jun 2. Exp Cell Res. 2020. PMID: 32502493
-
Hypoxia and angiogenesis in rheumatoid arthritis.Curr Opin Rheumatol. 2005 May;17(3):293-8. doi: 10.1097/01.bor.0000155361.83990.5b. Curr Opin Rheumatol. 2005. PMID: 15838239 Review.
-
The Role of Sumoylation in the Response to Hypoxia: An Overview.Cells. 2020 Oct 26;9(11):2359. doi: 10.3390/cells9112359. Cells. 2020. PMID: 33114748 Free PMC article. Review.
Cited by
-
The role of acetylation and deacetylation in cancer metabolism.Clin Transl Med. 2025 Jan;15(1):e70145. doi: 10.1002/ctm2.70145. Clin Transl Med. 2025. PMID: 39778006 Free PMC article. Review.
-
SUMOylation at the crossroads of gut health: insights into physiology and pathology.Cell Commun Signal. 2024 Aug 19;22(1):404. doi: 10.1186/s12964-024-01786-5. Cell Commun Signal. 2024. PMID: 39160548 Free PMC article. Review.
-
Role of ENO1 and its targeted therapy in tumors.J Transl Med. 2024 Nov 14;22(1):1025. doi: 10.1186/s12967-024-05847-8. J Transl Med. 2024. PMID: 39543641 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

