Possible role of lncRNAs in amelioration of Parkinson's disease symptoms by transplantation of dopaminergic cells
- PMID: 38472261
- PMCID: PMC10933336
- DOI: 10.1038/s41531-024-00661-x
Possible role of lncRNAs in amelioration of Parkinson's disease symptoms by transplantation of dopaminergic cells
Abstract
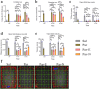
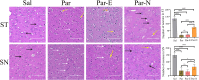
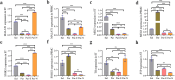
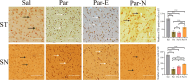
Long non-coding RNAs (lncRNAs) are biomarkers for diagnosis and treatment of Parkinson's disease (PD). Since dopaminergic cell transplantation is a clinical method to treat PD, this study investigated the effects of dopaminergic cell therapy on the expression of some lncRNAs and genes related to PD. In this study, Twenty-eight rats were randomly assigned to four experimental groups. The control group (Sal group) received saline injections. The Par group was a PD rat model with 6-hydroxydopamine (6-OHDA) injection in right striatum (ST). PD animals were transplanted by undifferentiated P19 stem cells (Par-E group), and P19-derived dopaminergic cells (Par-N group). Cell transplant effects were evaluated using behavioral tests (cylinder, open field, and rotarod tests), and histological methods (H&E and Nissl staining, and immunohistochemistry). Moreover, the expression of lncRNAs MALAT1, MEG3, and SNHG1, alongside specific neuronal (synaptophysin) and dopaminergic (tyrosine hydroxylase) markers was evaluated by qRT-PCR. Behavioral and histopathological examinations revealed that cell transplantation partially compensated dopaminergic cell degeneration in ST and substantia nigra (SN) of PD rats. The expression of MALAT1, SNHG1, and MEG3 was decreased in the ST of the Par group, while MEG3 and SNHG1 gene expression was increased in PBMC relative to the Sal group. In PBMC of the Par-N group, all three lncRNAs showed a reduction in their expression. Conversely, MALAT1 and SNHG1 expression was increased in ST tissue, while MEG3 gene expression was decreased compared to the Sal group. In conclusion, dopaminergic cell transplantation could change the lncRNAs expression. Furthermore, it partially improves symptoms in PD rats.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Expression variation of long noncoding RNAs in dopaminergic cells-derived from stem cells and their MPP+ induced PD models.Hum Mol Genet. 2025 Mar 20;34(7):599-610. doi: 10.1093/hmg/ddae192. Hum Mol Genet. 2025. PMID: 39820316
-
Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants.J Neurosci. 2001 May 15;21(10):3521-30. doi: 10.1523/JNEUROSCI.21-10-03521.2001. J Neurosci. 2001. PMID: 11331381 Free PMC article.
-
[Effect of electroacupuncture regulating NLRP3/Caspase-1 pathway on pyroptosis of dopaminergic neurons in rats with Parkinson's disease].Zhen Ci Yan Jiu. 2022 Nov 25;47(11):983-92. doi: 10.13702/j.1000-0607.20211016. Zhen Ci Yan Jiu. 2022. PMID: 36453675 Chinese.
-
Neural stem cells transplantation combined with ethyl stearate improve PD rats motor behavior by promoting NSCs migration and differentiation.CNS Neurosci Ther. 2023 Jun;29(6):1571-1584. doi: 10.1111/cns.14119. Epub 2023 Mar 16. CNS Neurosci Ther. 2023. PMID: 36924304 Free PMC article.
-
Long non-coding RNAs: From disease code to drug role.Acta Pharm Sin B. 2021 Feb;11(2):340-354. doi: 10.1016/j.apsb.2020.10.001. Epub 2020 Oct 10. Acta Pharm Sin B. 2021. PMID: 33643816 Free PMC article. Review.
Cited by
-
Potential Regulation of the Long Non-Coding RNA Metastasis-Associated Lung Adenocarcinoma Transcript1 by Estrogen in Parkinson's Disease.Life (Basel). 2024 Dec 16;14(12):1662. doi: 10.3390/life14121662. Life (Basel). 2024. PMID: 39768369 Free PMC article. Review.
References
-
- Maegawa, H. & Niwa, H. in Experimental Models of Parkinson’s Disease 95–110 (Springer, 2021).
LinkOut - more resources
Full Text Sources

