Twelve-hour normothermic liver perfusion in a rat model: characterization of the changes in the ex-situ bio-molecular phenotype and metabolism
- PMID: 38472309
- PMCID: PMC10933381
- DOI: 10.1038/s41598-024-56433-3
Twelve-hour normothermic liver perfusion in a rat model: characterization of the changes in the ex-situ bio-molecular phenotype and metabolism
Abstract
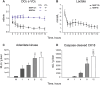
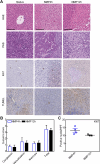
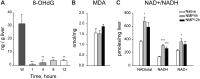
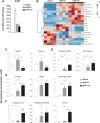
The partial understanding of the biological events that occur during normothermic machine perfusion (NMP) and particularly during prolonged perfusion might hinder its deployment in clinical transplantation. The aim of our study was to implement a rat model of prolonged NMP to characterize the bio-molecular phenotype and metabolism of the perfused organs. Livers (n = 5/group) were procured and underwent 4 h (NMP4h) or 12 h (NMP12h) NMP, respectively, using a perfusion fluid supplemented with an acellular oxygen carrier. Organs that were not exposed to any procedure served as controls (Native). All perfused organs met clinically derived viability criteria at the end of NMP. Factors related to stress-response and survival were increased after prolonged perfusion. No signs of oxidative damage were detected in both NMP groups. Evaluation of metabolite profiles showed preserved mitochondrial function, activation of Cori cycle, induction of lipolysis, acetogenesis and ketogenesis in livers exposed to 12 h-NMP. Increased concentrations of metabolites involved in glycogen synthesis, glucuronidation, bile acid conjugation, and antioxidant response were likewise observed. In conclusion, our NMP12h model was able to sustain liver viability and function, thereby deeply changing cell homeostasis to maintain a newly developed equilibrium. Our findings provide valuable information for the implementation of optimized protocols for prolonged NMP.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Quality Assessment by Bile Composition in Normothermic Machine Perfusion of Rat Livers.Tissue Eng Part A. 2025 Mar;31(5-6):244-254. doi: 10.1089/ten.TEA.2024.0048. Epub 2024 Jul 3. Tissue Eng Part A. 2025. PMID: 38832856
-
Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial.Ann Surg. 2019 Nov;270(5):906-914. doi: 10.1097/SLA.0000000000003540. Ann Surg. 2019. PMID: 31633615 Clinical Trial.
-
Normothermic Machine Perfusion Combined with Bone Marrow Mesenchymal Stem Cells Improves the Oxidative Stress Response and Mitochondrial Function in Rat Donation After Circulatory Death Livers.Stem Cells Dev. 2020 Jul 1;29(13):835-852. doi: 10.1089/scd.2019.0301. Epub 2020 May 5. Stem Cells Dev. 2020. PMID: 32253985 Free PMC article.
-
Normothermic ex-situ liver preservation: the new gold standard.Curr Opin Organ Transplant. 2017 Jun;22(3):274-280. doi: 10.1097/MOT.0000000000000414. Curr Opin Organ Transplant. 2017. PMID: 28333889 Review.
-
Normothermic liver preservation, current status and future directions.Curr Opin Organ Transplant. 2018 Jun;23(3):347-352. doi: 10.1097/MOT.0000000000000531. Curr Opin Organ Transplant. 2018. PMID: 29629996 Review.
Cited by
-
Role of Iron Metabolic Disturbances and Inflammatory Iron Biomarkers in Liver Transplant Prognosis.Int J Med Sci. 2025 Jul 1;22(13):3202-3219. doi: 10.7150/ijms.113479. eCollection 2025. Int J Med Sci. 2025. PMID: 40765572 Free PMC article. Review.
-
A proof-of-concept study in small and large animal models for coupling liver normothermic machine perfusion with mesenchymal stromal cell bioreactors.Nat Commun. 2025 Jan 2;16(1):283. doi: 10.1038/s41467-024-55217-7. Nat Commun. 2025. PMID: 39746966 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

