Prenatal dexamethasone exposure impairs rat blood-testis barrier function and sperm quality in adult offspring via GR/KDM1B/FSTL3/TGFβ signaling
- PMID: 38472317
- PMCID: PMC11130295
- DOI: 10.1038/s41401-024-01244-5
Prenatal dexamethasone exposure impairs rat blood-testis barrier function and sperm quality in adult offspring via GR/KDM1B/FSTL3/TGFβ signaling
Abstract
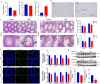
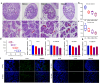
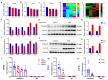
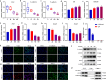
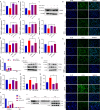
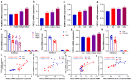
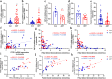
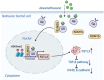
Both epidemiological and animal studies suggest that adverse environment during pregnancy can change the offspring development programming, but it is difficult to achieve prenatal early warning. In this study we investigated the impact of prenatal dexamethasone exposure (PDE) on sperm quality and function of blood-testis barrier (BTB) in adult offspring and the underlying mechanisms. Pregnant rats were injected with dexamethasone (0.1, 0.2 and 0.4 mg·kg-1·d-1, s.c.) from GD9 to GD20. After weaning (PW4), the pups were fed with lab chow. At PW12 and PW28, the male offspring were euthanized to collect blood and testes samples. We showed that PDE significantly decreased sperm quality (including quantity and motility) in male offspring, which was associated with impaired BTB and decreased CX43/E-cadherin expression in the testis. We demonstrated that PDE induced morphological abnormalities of fetal testicle and Sertoli cell development originated from intrauterine. By tracing to fetal testicular Sertoli cells, we found that PDE dose-dependently increased expression of histone lysine demethylases (KDM1B), decreasing histone 3 lysine 9 dimethylation (H3K9me2) levels of follistatin-like-3 (FSTL3) promoter region and increased FSTL3 expression, and inhibited TGFβ signaling and CX43/E-cadherin expression in offspring before and after birth. These results were validated in TM4 Sertoli cells following dexamethasone treatment. Meanwhile, the H3K9me2 levels of FSTL3 promoter in maternal peripheral blood mononuclear cell (PBMC) and placenta were decreased and its expression increased, which was positively correlated with the changes in offspring testis. Based on analysis of human samples, we found that the H3K9me2 levels of FSTL3 promoter in maternal blood PBMC and placenta were positively correlated with fetal blood testosterone levels after prenatal dexamethasone exposure. We conclude that PDE can reduce sperm quality in adult offspring rats, which is related to the damage of testis BTB via epigenetic modification and change of FSTL3 expression in Sertoli cells. The H3K9me2 levels of the FSTL3 promoter and its expression in the maternal blood PBMC can be used as a prenatal warning marker for fetal testicular dysplasia.
Keywords: FSTL3; H3K9me2; blood-testis barrier; prenatal dexamethasone exposure; prenatal warning markers; testicular dysplasia.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests. All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures
Similar articles
-
Semen Cuscutae-Fructus Lycii improves spermatogenic dysfunction by repairing the blood-testis barrier in rats according to in silico and in vitro methods.J Ethnopharmacol. 2021 Jun 28;274:114022. doi: 10.1016/j.jep.2021.114022. Epub 2021 Mar 16. J Ethnopharmacol. 2021. PMID: 33741439
-
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits: DART Report 07 [Internet].Research Triangle Park (NC): National Toxicology Program; 2022 Jan. Research Triangle Park (NC): National Toxicology Program; 2022 Jan. PMID: 35593777 Free Books & Documents. Review.
-
Polyethylene microplastics disrupt focal adhesion kinase (FAK) signaling and sertoli cell metabolism, compromising blood-testis barrier function and spermatogenesis.Toxicology. 2025 Nov;517:154240. doi: 10.1016/j.tox.2025.154240. Epub 2025 Jul 22. Toxicology. 2025. PMID: 40701262
-
Dexamethasone induces transgenerational inheritance of fetal-derived glomerulosclerosis phenotype in offspring through GR/DNMT3a mediated alterations of the lncRNA-Meg3/Notch signaling pathway.Cell Commun Signal. 2025 Jul 17;23(1):345. doi: 10.1186/s12964-025-02346-1. Cell Commun Signal. 2025. PMID: 40676611 Free PMC article.
-
Antioxidants for male subfertility.Cochrane Database Syst Rev. 2014;(12):CD007411. doi: 10.1002/14651858.CD007411.pub3. Epub 2014 Dec 15. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. PMID: 25504418 Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

