RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates
- PMID: 38472406
- PMCID: PMC11230912
- DOI: 10.1038/s41557-024-01467-3
RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates
Abstract
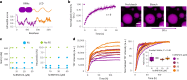
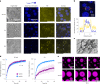
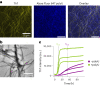
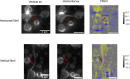
Several RNA binding proteins involved in membraneless organelles can form pathological amyloids associated with neurodegenerative diseases, but the mechanisms of how this aggregation is modulated remain elusive. Here we investigate how heterotypic protein-RNA interactions modulate the condensation and the liquid to amyloid transition of hnRNPA1A, a protein involved in amyothropic lateral sclerosis. In the absence of RNA, formation of condensates promotes hnRNPA1A aggregation and fibrils are localized at the interface of the condensates. Addition of RNA modulates the soluble to amyloid transition of hnRNPA1A according to different pathways depending on RNA/protein stoichiometry. At low RNA concentrations, RNA promotes both condensation and amyloid formation, and the catalytic effect of RNA adds to the role of the interface between the dense and dilute phases. At higher RNA concentrations, condensation is suppressed according to re-entrant phase behaviour but formation of hnRNPA1A amyloids is observed over longer incubation times. Our findings show how heterotypic nucleic acid-protein interactions affect the kinetics and molecular pathways of amyloid formation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The role of biomolecular condensates in protein aggregation.Nat Rev Chem. 2024 Sep;8(9):686-700. doi: 10.1038/s41570-024-00635-w. Epub 2024 Aug 12. Nat Rev Chem. 2024. PMID: 39134696 Review.
-
Initiation of hnRNPA1 Low-Complexity Domain Condensation Monitored by Dynamic Light Scattering.Int J Mol Sci. 2024 Jun 21;25(13):6825. doi: 10.3390/ijms25136825. Int J Mol Sci. 2024. PMID: 38999934 Free PMC article.
-
Chaperone-Mediated Heterotypic Phase Separation Prevents the Amyloid Formation of the Pathological Y145Stop Prion Protein Variant.J Mol Biol. 2025 Mar 1;437(5):168955. doi: 10.1016/j.jmb.2025.168955. Epub 2025 Jan 16. J Mol Biol. 2025. PMID: 39826709
-
The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils.Nat Chem. 2023 Oct;15(10):1340-1349. doi: 10.1038/s41557-023-01289-9. Epub 2023 Sep 25. Nat Chem. 2023. PMID: 37749234 Free PMC article.
-
Splicing regulation through biomolecular condensates and membraneless organelles.Nat Rev Mol Cell Biol. 2024 Sep;25(9):683-700. doi: 10.1038/s41580-024-00739-7. Epub 2024 May 21. Nat Rev Mol Cell Biol. 2024. PMID: 38773325 Free PMC article. Review.
Cited by
-
Unraveling the Bivalent and Rapid Interactions Between a Multivalent RNA Recognition Motif and RNA: A Kinetic Approach.Biochemistry. 2024 Nov 5;63(21):2816-2829. doi: 10.1021/acs.biochem.4c00301. Epub 2024 Oct 14. Biochemistry. 2024. PMID: 39397705 Free PMC article.
-
Harnessing Competitive Interactions to Regulate Supramolecular "Micelle-Droplet-Fiber" Transition and Reversibility in Water.J Am Chem Soc. 2024 Oct 30;146(43):29759-29766. doi: 10.1021/jacs.4c11285. Epub 2024 Oct 15. J Am Chem Soc. 2024. PMID: 39405510 Free PMC article.
-
Enhancement of enzymatic activity by biomolecular condensates through pH buffering.Nat Commun. 2025 Jul 10;16(1):6368. doi: 10.1038/s41467-025-61013-8. Nat Commun. 2025. PMID: 40640131 Free PMC article.
-
Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates.Viruses. 2025 Jan 13;17(1):97. doi: 10.3390/v17010097. Viruses. 2025. PMID: 39861886 Free PMC article.
-
Aging-dependent evolving electrochemical potentials of biomolecular condensates regulate their physicochemical activities.Nat Chem. 2025 May;17(5):756-766. doi: 10.1038/s41557-025-01762-7. Epub 2025 Mar 12. Nat Chem. 2025. PMID: 40074825
References
-
- Gebauer, F., Schwarzl, T., Valcárcel, J. & Hentze, M. W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet.22, 185–198 (2021). - PubMed
-
- Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science314, 130–133 (2006). - PubMed
-
- Rademakers, R., Neumann, M., Mackenzie, I. R. A. & Rademakers, R. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol.9, 995–1007 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

