A Low Dose of Rapamycin Promotes Hair Cell Differentiation by Enriching SOX2+ Progenitors in the Neonatal Mouse Inner Ear Organoids
- PMID: 38472516
- PMCID: PMC11018585
- DOI: 10.1007/s10162-024-00938-1
A Low Dose of Rapamycin Promotes Hair Cell Differentiation by Enriching SOX2+ Progenitors in the Neonatal Mouse Inner Ear Organoids
Abstract
Purpose: To investigate the impact of rapamycin on the differentiation of hair cells.
Methods: Murine cochlear organoids were derived from cochlear progenitor cells. Different concentrations of rapamycin were added into the culture medium at different proliferation and differentiation stages.
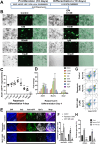
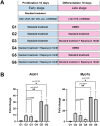
Results: Rapamycin exhibited a concentration-dependent reduction in the proliferation of these inner ear organoids. Nevertheless, organoids subjected to a 10-nM dose of rapamycin demonstrated a markedly increased proportion of hair cells. Furthermore, rapamycin significantly upregulated the expression of markers associated with both hair cells and supporting cells, including ATOH1, MYO7A, and SOX2. Mechanistic studies revealed that rapamycin preferentially suppressed cells without Sox2 expression during the initial proliferation stage, thereby augmenting and refining the population of SOX2+ progenitors. These enriched progenitors were predisposed to differentiate into hair cells during the later stages of organoid development. Conversely, the use of the mTOR activator MHY 1485 demonstrated opposing effects.
Conclusion: Our findings underscore a practical strategy for enhancing the generation of inner ear organoids with a low dose of rapamycin, achieved by enriching SOX2+ progenitors in an in vitro setting.
Keywords: Hair cell; Inner ear; Organoids; Rapamycin; Regeneration.
© 2024. The Author(s) under exclusive licence to Association for Research in Otolaryngology.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1.PLoS One. 2012;7(1):e30871. doi: 10.1371/journal.pone.0030871. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292066 Free PMC article.
-
Optimized inner ear organoids for efficient hair cell generation and ototoxicity response modeling.Sci China Life Sci. 2025 May;68(5):1369-1383. doi: 10.1007/s11427-024-2803-1. Epub 2025 Jan 23. Sci China Life Sci. 2025. PMID: 39862345
-
Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture.PLoS One. 2016 Sep 8;11(9):e0162508. doi: 10.1371/journal.pone.0162508. eCollection 2016. PLoS One. 2016. PMID: 27607106 Free PMC article.
-
Sox2 regulation of hair cell development: incoherence makes sense.Hear Res. 2013 Mar;297:20-9. doi: 10.1016/j.heares.2012.11.003. Epub 2012 Nov 12. Hear Res. 2013. PMID: 23154195 Review.
-
Recent Progress in Generation of Inner Ear Organoid.Adv Biol (Weinh). 2024 Oct;8(10):e2400223. doi: 10.1002/adbi.202400223. Epub 2024 Jul 25. Adv Biol (Weinh). 2024. PMID: 39051423 Review.
Cited by
-
Determination of Differential miRNA Expression Profile in People with Noise-Induced Hearing Loss.Int J Mol Sci. 2025 Jul 10;26(14):6623. doi: 10.3390/ijms26146623. Int J Mol Sci. 2025. PMID: 40724871 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

