Real-world Impact of Fremanezumab on Migraine-Related Health Care Resource Utilization in Patients with Comorbidities, Acute Medication Overuse, and/or Unsatisfactory Prior Migraine Preventive Response
- PMID: 38472655
- PMCID: PMC11111425
- DOI: 10.1007/s40122-024-00583-9
Real-world Impact of Fremanezumab on Migraine-Related Health Care Resource Utilization in Patients with Comorbidities, Acute Medication Overuse, and/or Unsatisfactory Prior Migraine Preventive Response
Abstract
Introduction: Fremanezumab, a humanized monoclonal antibody targeting calcitonin gene-related peptide, is indicated for preventive treatment of migraine in adults. Real-world evidence assessing the effect of fremanezumab on migraine-related medication use, health care resource utilization (HCRU), and costs in patient populations with comorbidities, acute medication overuse (AMO), and/or unsatisfactory prior migraine preventive response (UPMPR) is needed.
Methods: Data for this US, retrospective claims analysis were obtained from the Merative® MarketScan® Commercial and supplemental databases. Eligible adults with migraine initiated fremanezumab between 1 September 2018 and 30 June 2019 (date of earliest fremanezumab claim is the index date), had ≥ 12 months of continuous enrollment prior to initiation (preindex period) and ≥ 6 months of data following initiation (postindex period; variable follow-up after 6 months), and had certain preindex migraine comorbidities (depression, anxiety, and cardiovascular disease), potential AMO, or UPMPR. Changes in migraine-related concomitant acute and preventive medication use, HCRU, and costs were assessed pre- versus postindex.
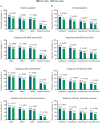
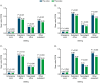
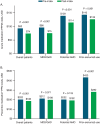
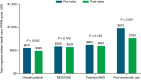
Results: In total, 3193 patients met the eligibility criteria. From pre- to postindex, mean (SD) per patient per month (PPPM) number of migraine-related acute medication and preventive medication claims (excluding fremanezumab), respectively, decreased from 0.97 (0.90) to 0.86 (0.87) (P < 0.001) and 0.94 (0.74) to 0.81 (0.75) (P < 0.001). Migraine-related outpatient and neurologist office visits, emergency department visits, and other outpatient services PPPM decreased pre- versus postindex (P < 0.001 for all), resulting in a reduction in mean (SD) total health care costs PPPM from US$541 (US$858) to US$490 (US$974) (P = 0.003). Patients showed high adherence and persistence rates, with mean (SD) proportion of days covered of 0.71 (0.29), medication possession ratio of 0.74 (0.31), and persistence duration of 160.3 (33.2) days 6 months postindex.
Conclusions: Patients with certain migraine comorbidities, potential AMO, and/or UPMPR in a real-world setting had reduced migraine-related medication use, HCRU, and costs following initiation of fremanezumab. Graphical abstract available for this article.
Keywords: Acute medication overuse; Adherence; CGRP; Costs; Fremanezumab; HCRU; Migraine; Real-world evidence.
© 2024. The Author(s).
Conflict of interest statement
Dawn C. Buse has received research and/or consulting funding from Allergan/Abbvie, Amgen, Biohaven, Collegium, Lilly, Lundbeck, and Teva Pharmaceutical Industries. Maurice T. Driessen, Lynda J. Krasenbaum, and Mario Ortega are employees of Teva Pharmaceutical Industries. Elizabeth R. Packnett is an employee of Merative (IBM Watson Health at the time the study was conducted), which was contracted by Teva Pharmaceutical Industries to conduct this study. Michael J. Seminerio and Karen Carr were employees of Teva Pharmaceutical Industries at the time of the study but have since left the company; they are currently employees of AbbVie.
Figures


Similar articles
-
Real-world impact of fremanezumab on migraine symptoms and resource utilization in the United States.J Headache Pain. 2021 Dec 20;22(1):156. doi: 10.1186/s10194-021-01358-9. J Headache Pain. 2021. PMID: 34930112 Free PMC article.
-
Health care resource utilization and direct costs incurred over 12 months by patients with migraine initiating self-injectable calcitonin gene-related peptide monoclonal antibodies: A US real-world study.J Manag Care Spec Pharm. 2025 Apr;31(4):351-365. doi: 10.18553/jmcp.2025.31.4.351. J Manag Care Spec Pharm. 2025. PMID: 40152794 Free PMC article.
-
US Real-World Effectiveness, Tolerability, and Healthcare Resource Utilization After Addition of Fremanezumab for Preventive Treatment in Patients Using Gepants for Acute Treatment of Migraine: Results From a Retrospective Chart Review.Adv Ther. 2025 Feb;42(2):1207-1221. doi: 10.1007/s12325-024-03063-w. Epub 2025 Jan 8. Adv Ther. 2025. PMID: 39775579 Free PMC article.
-
Fremanezumab for the Treatment of Migraine Complicated by Medication Overuse: A Systematic Review.Clin Drug Investig. 2025 May;45(5):247-254. doi: 10.1007/s40261-025-01433-y. Epub 2025 Mar 22. Clin Drug Investig. 2025. PMID: 40121372
-
Fremanezumab: a disease-specific option for the preventive treatment of migraine, including difficult-to-treat migraine.Emerg Top Life Sci. 2020 Sep 8;4(2):179-190. doi: 10.1042/ETLS20200018. Emerg Top Life Sci. 2020. PMID: 32832978 Free PMC article. Review.
Cited by
-
Real-World Lessons with Fremanezumab as the Third Available CGRP Monoclonal Antibody in a Third-Level Hospital: Focus on the Factors Predicting Response.J Clin Med. 2025 Feb 7;14(4):1054. doi: 10.3390/jcm14041054. J Clin Med. 2025. PMID: 40004586 Free PMC article.
References
-
- Buse DC, Reed ML, Fanning KM, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2020;21(1):23. doi: 10.1186/s10194-020-1084-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

