Cyclosporin A-loaded dissolving microneedles for dermatitis therapy: Development, characterisation and efficacy in a delayed-type hypersensitivity in vivo model
- PMID: 38472726
- PMCID: PMC11499354
- DOI: 10.1007/s13346-024-01542-9
Cyclosporin A-loaded dissolving microneedles for dermatitis therapy: Development, characterisation and efficacy in a delayed-type hypersensitivity in vivo model
Abstract
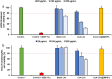
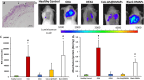
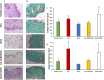
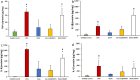
Several drugs can be used for treating inflammatory skin pathologies like dermatitis and psoriasis. However, for the management of chronic and long-term cases, topical administration is preferred over oral delivery since it prevents certain issues due to systemic side effects from occurring. Cyclosporin A (CsA) has been used for this purpose; however, its high molecular weight (1202 Da) restricts the diffusion through the skin structure. Here, we developed a nano-in-micro device combining lipid vesicles (LVs) and dissolving microneedle array patches (DMAPs) for targeted skin delivery. CsA-LVs allowed the effective incorporation of CsA in the hydrophilic DMAP matrix despite the hydrophobicity of the drug. Polymeric matrix composed of poly (vinyl alcohol) (5% w/v), poly (vinyl pyrrolidine) (15% w/v) and CsA-LV dispersion (10% v/v) led to the formation of CsA-LVs@DMAPs with adequate mechanical properties to penetrate the stratum corneum barrier. The safety and biocompatibility were ensured in an in vitro viability test using HaCaT keratinocytes and L929 fibroblast cell lines. Ex vivo permeability studies in a Franz-diffusion cell setup showed effective drug retention in the skin structure. Finally, CsA-LVs@DMAPs were challenged in an in vivo murine model of delayed-type hypersensitivity to corroborate their potential to ameliorate skin inflammatory conditions. Different findings like photon emission reduction in bioluminescence study, normalisation of histological damage and decrease of inflammatory cytokines point out the effectivity of CsA-LVs@DMAPs to treat these conditions. Overall, our study demonstrates that CsA-LVs@DMAPs can downregulate the skin inflammatory environment which paves the way for their clinical translation and their use as an alternative to corticosteroid-based therapies.
Keywords: Cyclosporin A; Dermatitis; Dissolving microneedles; Lipid vesicles; Skin delivery; Skin inflammatory conditions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Transdermal delivery of enfuvirtide using dissolving microneedles integrated with novel insertion and removal indicator.J Control Release. 2025 Sep 10;385:113954. doi: 10.1016/j.jconrel.2025.113954. Epub 2025 Jun 16. J Control Release. 2025. PMID: 40532764
-
Cubosome-dissolving microneedle system enhances the anti-psoriatic efficacy of a synergistic combination of methotrexate and cyclosporine.Int J Pharm. 2025 Aug 20;681:125893. doi: 10.1016/j.ijpharm.2025.125893. Epub 2025 Jun 24. Int J Pharm. 2025. PMID: 40571200
-
Topical cyclosporine for atopic keratoconjunctivitis.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009078. doi: 10.1002/14651858.CD009078.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972132 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
(Re)evolution in nanoparticles-loaded microneedle delivery systems: are we getting closer to a clinical translation?Nanomedicine (Lond). 2025 May;20(10):1195-1207. doi: 10.1080/17435889.2025.2492538. Epub 2025 Apr 21. Nanomedicine (Lond). 2025. PMID: 40257286 Review.
-
Novel drug delivery systems in topical treatment of atopic dermatitis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):9851-9872. doi: 10.1007/s00210-025-04002-4. Epub 2025 Mar 13. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40080153 Review.
-
Recent advances and perspectives of MicroNeedles for biomedical applications.Biophys Rev. 2025 Apr 24;17(3):909-928. doi: 10.1007/s12551-025-01317-7. eCollection 2025 Jun. Biophys Rev. 2025. PMID: 40727662 Free PMC article. Review.
References
-
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:8–16. - PubMed
-
- Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18–25. - PubMed
-
- Thyssen JP, Halling A-S, Schmid-Grendelmeier P, Guttman-Yassky E, Silverberg JI. Comorbidities of atopic dermatitis-what does the evidence say? J Allergy Clin Immunol. 2023;151:1155–62. - PubMed
-
- Siegels D, Heratizadeh A, Abraham S, Binnmyr J, Brockow K, Irvine AD, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta-analysis. Allergy Eur J Allergy Clin Immunol. 2021;76:1053–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

