Integrating Artificial Intelligence for Advancing Multiple-Cancer Early Detection via Serum Biomarkers: A Narrative Review
- PMID: 38473224
- PMCID: PMC10931531
- DOI: 10.3390/cancers16050862
Integrating Artificial Intelligence for Advancing Multiple-Cancer Early Detection via Serum Biomarkers: A Narrative Review
Abstract
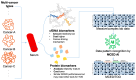
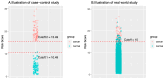
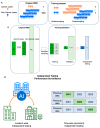
The concept and policies of multicancer early detection (MCED) have gained significant attention from governments worldwide in recent years. In the era of burgeoning artificial intelligence (AI) technology, the integration of MCED with AI has become a prevailing trend, giving rise to a plethora of MCED AI products. However, due to the heterogeneity of both the detection targets and the AI technologies, the overall diversity of MCED AI products remains considerable. The types of detection targets encompass protein biomarkers, cell-free DNA, or combinations of these biomarkers. In the development of AI models, different model training approaches are employed, including datasets of case-control studies or real-world cancer screening datasets. Various validation techniques, such as cross-validation, location-wise validation, and time-wise validation, are used. All of the factors show significant impacts on the predictive efficacy of MCED AIs. After the completion of AI model development, deploying the MCED AIs in clinical practice presents numerous challenges, including presenting the predictive reports, identifying the potential locations and types of tumors, and addressing cancer-related information, such as clinical follow-up and treatment. This study reviews several mature MCED AI products currently available in the market, detecting their composing factors from serum biomarker detection, MCED AI training/validation, and the clinical application. This review illuminates the challenges encountered by existing MCED AI products across these stages, offering insights into the continued development and obstacles within the field of MCED AI.
Keywords: AI; multi-cancer early detection (MCED); serum biomarkers.
Conflict of interest statement
H.-Y. Wang has a financial interest in the licensed technology related to this work, as it has been licensed to 20/20 GeneSystems. Additionally, C. Zhou and M. Lebowitz are employed by 20/20 GeneSystems.
Figures

References
-
- Zutshi V., Kaur G. Remembering George Papanicolaou: A Revolutionary Who Invented the Pap Smear Test. J. Colposc. Low. Genit. Tract. Pathol. 2023;1:47–49.
Publication types
LinkOut - more resources
Full Text Sources

