Prospective Assessment of Fluorine-18-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) for Early Identification of Checkpoint-Inhibitor-Induced Pseudoprogression
- PMID: 38473325
- PMCID: PMC10931278
- DOI: 10.3390/cancers16050964
Prospective Assessment of Fluorine-18-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) for Early Identification of Checkpoint-Inhibitor-Induced Pseudoprogression
Abstract
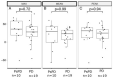
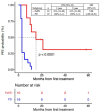
The activity of immune checkpoint inhibitors (ICIs) in patients with metastatic melanoma is often monitored using fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) scans. However, distinguishing disease progression (PD) from pseudoprogression (PsPD), where increased FDG uptake might reflect immune cell activity rather than tumor growth, remains a challenge. This prospective study compared the efficacy of dual-time point (DTP) FDG-PET/CT with modified response criteria (PERCIMT) in differentiating PsPD from PD. From July 2017-January 2021, 41 patients suspected to have PsPD on an evaluation scan were prospectively included (29 evaluable). A subsequent DTP FDG-PET/CT scan was conducted within 14 days, followed by a confirmatory FDG-PET/CT scan. Additionally, PERCIMT were applied. DTP FDG-PET/CT identified 24% with PsPD and 76% with PD. Applying PERCIMT criteria, 69% showed PsPD, while 31% had PD. On follow-up, 10 patients (34%) demonstrated confirmed PsPD, while 19 (66%) exhibited PD. The sensitivity and specificity of DTP FDG-PET/CT were 20% and 74%, respectively, and for PERCIMT this was 80% and 37%, respectively. Our findings suggest limited efficacy of DTP FDG-PET/CT in distinguishing PsPD from PD in ICI-treated patients with metastatic melanoma. The use of PERCIMT could complement clinical assessment and be incorporated in multidisciplinary team conferences for enhanced decision-making.
Keywords: FDG-PET/CT; PERCIMT; dual-time point FDG-PET/CT; immune checkpoint inhibitors; metastatic melanoma; pseudoprogression.
Conflict of interest statement
EE received honoraria for consultancies and lectures from Novartis, Merck, Bristol-Myers Squibb, and Pierre Fabre, and conference and travel support from Pierre Fabre and Merck. MD received access to proprietary data from Bristol Myers Squibb, and Genentech and is advisor of Achilles Therapeutics. IMS has received honoraria for consultancies and lectures from Novartis, Roche, Merck, and Bristol Myers Squibb; a restricted research grant from Novartis; and financial support for attending symposia from Bristol Myers Squibb, Merck, Novartis, Pfizer, and Roche. The other authors declare no conflicts of interest.
Figures




Similar articles
-
Predictive value and accuracy of [18F]FDG PET/CT modified response criteria for checkpoint immunotherapy in patients with advanced melanoma.Eur J Nucl Med Mol Imaging. 2023 Jul;50(9):2715-2726. doi: 10.1007/s00259-023-06247-8. Epub 2023 May 4. Eur J Nucl Med Mol Imaging. 2023. PMID: 37140669 Free PMC article.
-
Assessment of early metabolic progression in melanoma patients under immunotherapy: an 18F-FDG PET/CT study.EJNMMI Res. 2021 Sep 8;11(1):89. doi: 10.1186/s13550-021-00832-4. EJNMMI Res. 2021. PMID: 34495433 Free PMC article.
-
Interim [18F]FDG PET/CT can predict response to anti-PD-1 treatment in metastatic melanoma.Eur J Nucl Med Mol Imaging. 2021 Jun;48(6):1932-1943. doi: 10.1007/s00259-020-05137-7. Epub 2020 Dec 18. Eur J Nucl Med Mol Imaging. 2021. PMID: 33336264 Free PMC article.
-
Monitoring of patients with metastatic melanoma treated with immune checkpoint inhibitors using PET-CT.Cancer Immunol Immunother. 2019 May;68(5):813-822. doi: 10.1007/s00262-018-2229-6. Epub 2018 Aug 19. Cancer Immunol Immunother. 2019. PMID: 30123922 Free PMC article. Review.
-
Tumor response assessment on imaging following immunotherapy.Front Oncol. 2022 Oct 25;12:982983. doi: 10.3389/fonc.2022.982983. eCollection 2022. Front Oncol. 2022. PMID: 36387133 Free PMC article. Review.
Cited by
-
Comparison of PERCIST5, imPERCIST5, and PERCIMT Criteria for Early Assessment of Pembrolizumab Response with FDG-PET/CT in Metastatic Bladder Cancer Patients.Pharmaceuticals (Basel). 2025 May 9;18(5):701. doi: 10.3390/ph18050701. Pharmaceuticals (Basel). 2025. PMID: 40430520 Free PMC article.
References
-
- Schina A., Pedersen S., Spenning A.L., Laursen O.K., Pedersen C., Haslund C.A., Schmidt H., Bastholt L., Svane I.M., Ellebaek E., et al. Sustained improved survival of patients with metastatic melanoma after the introduction of anti-PD-1-based therapies. Eur. J. Cancer. 2023;195:113392. doi: 10.1016/j.ejca.2023.113392. - DOI - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
-
- Aide N., Hicks R.J., Le Tourneau C., Lheureux S., Fanti S., Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:238–250. doi: 10.1007/s00259-018-4171-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

