Prospective Assessment of Fluorine-18-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) for Early Identification of Checkpoint-Inhibitor-Induced Pseudoprogression
- PMID: 38473325
- PMCID: PMC10931278
- DOI: 10.3390/cancers16050964
Prospective Assessment of Fluorine-18-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) for Early Identification of Checkpoint-Inhibitor-Induced Pseudoprogression
Abstract
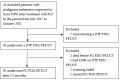
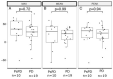
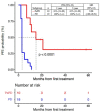
The activity of immune checkpoint inhibitors (ICIs) in patients with metastatic melanoma is often monitored using fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) scans. However, distinguishing disease progression (PD) from pseudoprogression (PsPD), where increased FDG uptake might reflect immune cell activity rather than tumor growth, remains a challenge. This prospective study compared the efficacy of dual-time point (DTP) FDG-PET/CT with modified response criteria (PERCIMT) in differentiating PsPD from PD. From July 2017-January 2021, 41 patients suspected to have PsPD on an evaluation scan were prospectively included (29 evaluable). A subsequent DTP FDG-PET/CT scan was conducted within 14 days, followed by a confirmatory FDG-PET/CT scan. Additionally, PERCIMT were applied. DTP FDG-PET/CT identified 24% with PsPD and 76% with PD. Applying PERCIMT criteria, 69% showed PsPD, while 31% had PD. On follow-up, 10 patients (34%) demonstrated confirmed PsPD, while 19 (66%) exhibited PD. The sensitivity and specificity of DTP FDG-PET/CT were 20% and 74%, respectively, and for PERCIMT this was 80% and 37%, respectively. Our findings suggest limited efficacy of DTP FDG-PET/CT in distinguishing PsPD from PD in ICI-treated patients with metastatic melanoma. The use of PERCIMT could complement clinical assessment and be incorporated in multidisciplinary team conferences for enhanced decision-making.
Keywords: FDG-PET/CT; PERCIMT; dual-time point FDG-PET/CT; immune checkpoint inhibitors; metastatic melanoma; pseudoprogression.
Conflict of interest statement
EE received honoraria for consultancies and lectures from Novartis, Merck, Bristol-Myers Squibb, and Pierre Fabre, and conference and travel support from Pierre Fabre and Merck. MD received access to proprietary data from Bristol Myers Squibb, and Genentech and is advisor of Achilles Therapeutics. IMS has received honoraria for consultancies and lectures from Novartis, Roche, Merck, and Bristol Myers Squibb; a restricted research grant from Novartis; and financial support for attending symposia from Bristol Myers Squibb, Merck, Novartis, Pfizer, and Roche. The other authors declare no conflicts of interest.
Figures




References
-
- Schina A., Pedersen S., Spenning A.L., Laursen O.K., Pedersen C., Haslund C.A., Schmidt H., Bastholt L., Svane I.M., Ellebaek E., et al. Sustained improved survival of patients with metastatic melanoma after the introduction of anti-PD-1-based therapies. Eur. J. Cancer. 2023;195:113392. doi: 10.1016/j.ejca.2023.113392. - DOI - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
-
- Aide N., Hicks R.J., Le Tourneau C., Lheureux S., Fanti S., Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:238–250. doi: 10.1007/s00259-018-4171-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

