Frontline and Relapsed Rhabdomyosarcoma (FaR-RMS) Clinical Trial: A Report from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG)
- PMID: 38473359
- PMCID: PMC10931395
- DOI: 10.3390/cancers16050998
Frontline and Relapsed Rhabdomyosarcoma (FaR-RMS) Clinical Trial: A Report from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG)
Erratum in
-
Correction: Chisholm et al. Frontline and Relapsed Rhabdomyosarcoma (FaR-RMS) Clinical Trial: A Report from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancers 2024, 16, 998.Cancers (Basel). 2024 Oct 9;16(19):3427. doi: 10.3390/cancers16193427. Cancers (Basel). 2024. PMID: 39410054 Free PMC article.
Abstract
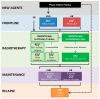
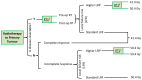
The Frontline and Relapsed Rhabdomyosarcoma (FaR-RMS) clinical trial is an overarching, multinational study for children and adults with rhabdomyosarcoma (RMS). The trial, developed by the European Soft Tissue Sarcoma Study Group (EpSSG), incorporates multiple different research questions within a multistage design with a focus on (i) novel regimens for poor prognostic subgroups, (ii) optimal duration of maintenance chemotherapy, and (iii) optimal use of radiotherapy for local control and widespread metastatic disease. Additional sub-studies focusing on biological risk stratification, use of imaging modalities, including [18F]FDG PET-CT and diffusion-weighted MRI imaging (DWI) as prognostic markers, and impact of therapy on quality of life are described. This paper forms part of a Special Issue on rhabdomyosarcoma and outlines the study background, rationale for randomisations and sub-studies, design, and plans for utilisation and dissemination of results.
Keywords: EpSSG; FaR-RMS; chemotherapy; clinical trial; novel agents; radiotherapy; randomisation; rhabdomyosarcoma.
Conflict of interest statement
JCC is supported by The Giant Pledge through the Royal Marsden Cancer Charity. This work represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London and the NIHR Birmingham Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. SAG has/has had an advisory role for EMD Serono/MERCK KGaA, AMGEN, and GILEAD; signed a consultancy agreement with AstraZeneca and Schroedinger Therapeutics; and received research funding (institution) from AstraZeneca, GSK, and BAYER. MC has had advisory and invited speaker roles for Bayer. SW leads the charity, Alice’s Arc which is funding the biological studies. PC is a member of the Advisory Board for AstraZeneca. JHMM has consultancy agreements with Merck, GSK and BAYER. BAYER has provided funding to the University of Birmingham for the FaR-RMS study.
Figures
References
-
- FaR-RMS: An Overarching Study for Children and Adults with Frontline and Relapsed RhabdoMyoSarcoma—Full Text View—ClinicalTrials.gov. [(accessed on 21 February 2024)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04625907.
-
- Hallpike E. Creative participation for young cancer survivors: Designing a logo for the European paediatric Soft tissue sarcoma Study Group (EpSSG) Frontline and Relapse-Rhabdomyosarcoma Study (FaR-RMS); Proceedings of the 4th Global AYA Conference; London, UK. 13–15 July 2020.
-
- [(accessed on 21 February 2024)]. Available online: https://www.alicesarc.org/
Grants and funding
LinkOut - more resources
Full Text Sources