Recent Advances in Therapeutic Strategies to Improve Colorectal Cancer Treatment
- PMID: 38473386
- PMCID: PMC10930828
- DOI: 10.3390/cancers16051029
Recent Advances in Therapeutic Strategies to Improve Colorectal Cancer Treatment
Abstract
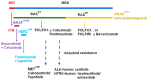
Colorectal cancer (CRC) is the second-leading cause of cancer-related mortality worldwide. CRC mortality results almost exclusively from metastatic disease (mCRC) for which systemic chemotherapy is often a preferred therapeutic option. Biomarker-based stratification of mCRC enables the use of precision therapy based on individual tumor mutational profiles. Activating mutations in the RAS/RAF/MAPK pathway downstream of EGFR signaling have, until recently, limited the use of EGFR-targeted therapies for mCRC; however, the development of anti-RAS and anti-RAF therapies together with improved strategies to limit compensatory signaling pathways is resulting in improved survival rates in several highly lethal mCRC sub-types (e.g., BRAF-mutant). The use of fluoropyrimidine (FP)-based chemotherapy regimens to treat mCRC continues to evolve contributing to improved long-term survival. Future advances in chemotherapy for mCRC will need to position development relative to the advances made in precision oncology.
Keywords: chemotherapy precision oncology; colorectal cancer; fluoropyrimidine.
Conflict of interest statement
The author declares no conflicts of interest.
Figures


References
-
- Innocenti F., Ou F.S., Qu X., Zemla T.J., Niedzwiecki D., Tam R., Mahajan S., Goldberg R.M., Bertagnolli M.M., Blanke C.D., et al. Mutational Analysis of Patients with Colorectal Cancer in CALGB/SWOG 80405 Identifies New Roles of Microsatellite Instability and Tumor Mutational Burden for Patient Outcome. J. Clin. Oncol. 2019;37:1217–1227. doi: 10.1200/JCO.18.01798. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

