Peptide Therapeutics: Unveiling the Potential against Cancer-A Journey through 1989
- PMID: 38473389
- PMCID: PMC11326481
- DOI: 10.3390/cancers16051032
Peptide Therapeutics: Unveiling the Potential against Cancer-A Journey through 1989
Abstract
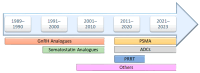
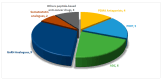
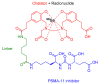
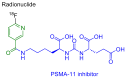
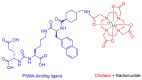
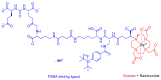
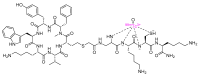
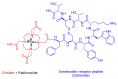
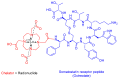
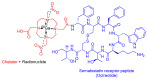
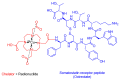
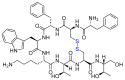
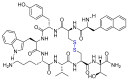
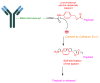
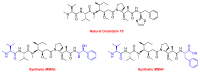
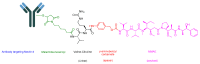
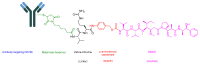
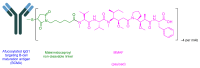
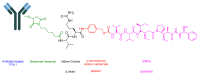
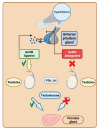
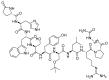
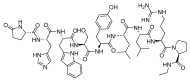
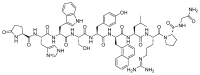
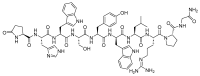
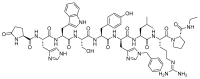
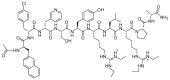
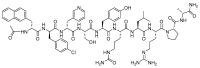
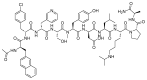
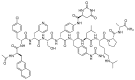
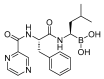
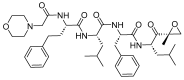
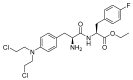
The United States Food and Drug Administration (FDA) has approved a plethora of peptide-based drugs as effective drugs in cancer therapy. Peptides possess high specificity, permeability, target engagement, and a tolerable safety profile. They exhibit selective binding with cell surface receptors and proteins, functioning as agonists or antagonists. They also serve as imaging agents for diagnostic applications or can serve a dual-purpose as both diagnostic and therapeutic (theragnostic) agents. Therefore, they have been exploited in various forms, including linkers, peptide conjugates, and payloads. In this review, the FDA-approved prostate-specific membrane antigen (PSMA) peptide antagonists, peptide receptor radionuclide therapy (PRRT), somatostatin analogs, antibody-drug conjugates (ADCs), gonadotropin-releasing hormone (GnRH) analogs, and other peptide-based anticancer drugs are analyzed in terms of their chemical structures and properties, therapeutic targets and mechanisms of action, development journey, administration routes, and side effects.
Keywords: ADC; PDC; antineoplastic; cancer; chemotherapy; drugs; imaging; oncology; peptides; theragnostic; tumor.
Conflict of interest statement
The author declares no conflicts of interest.
Figures


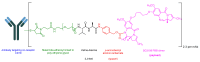
Similar articles
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
-
Update: improvement strategies for peptide receptor scintigraphy and radionuclide therapy.Cancer Biother Radiopharm. 2008 Apr;23(2):137-57. doi: 10.1089/cbr.2007.0435. Cancer Biother Radiopharm. 2008. PMID: 18454684 Review.
-
Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors.Eur J Endocrinol. 1999 Jul;141(1):1-14. doi: 10.1530/eje.0.1410001. Eur J Endocrinol. 1999. PMID: 10407215 Review.
-
Development, efficacy and side effects of antibody‑drug conjugates for cancer therapy (Review).Mol Clin Oncol. 2023 May 4;18(6):47. doi: 10.3892/mco.2023.2643. eCollection 2023 Jun. Mol Clin Oncol. 2023. PMID: 37206431 Free PMC article. Review.
-
Stepping forward in antibody-drug conjugate development.Pharmacol Ther. 2022 Jan;229:107917. doi: 10.1016/j.pharmthera.2021.107917. Epub 2021 Jun 24. Pharmacol Ther. 2022. PMID: 34171334 Free PMC article. Review.
Cited by
-
Elastin-Derived Peptide-Based Hydrogels as a Potential Drug Delivery System.Gels. 2024 Aug 12;10(8):531. doi: 10.3390/gels10080531. Gels. 2024. PMID: 39195060 Free PMC article.
-
Peptidergic Systems and Neuroblastoma.Int J Mol Sci. 2025 Apr 8;26(8):3464. doi: 10.3390/ijms26083464. Int J Mol Sci. 2025. PMID: 40331938 Free PMC article. Review.
-
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer: Current Use and Future Prospects.Int J Mol Sci. 2024 Sep 17;25(18):10008. doi: 10.3390/ijms251810008. Int J Mol Sci. 2024. PMID: 39337496 Free PMC article. Review.
-
FDA-Approved Antibacterials and Echinocandins.Antibiotics (Basel). 2025 Feb 7;14(2):166. doi: 10.3390/antibiotics14020166. Antibiotics (Basel). 2025. PMID: 40001410 Free PMC article. Review.
-
Peptide-Drug Conjugates: A New Hope for Cancer.J Pept Sci. 2025 Aug;31(8):e70040. doi: 10.1002/psc.70040. J Pept Sci. 2025. PMID: 40646707 Free PMC article. Review.
References
-
- Curtius T. Ueber einige neue der Hippursäure analog constituirte, synthetisch dargestellte Amidosäuren. J. Prakt. Chem. 1882;26:145–208. doi: 10.1002/prac.18820260112. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

