Haploinsufficiency of Adenomatous Polyposis Coli Coupled with Kirsten Rat Sarcoma Viral Oncogene Homologue Activation and P53 Loss Provokes High-Grade Glioblastoma Formation in Mice
- PMID: 38473403
- PMCID: PMC10930734
- DOI: 10.3390/cancers16051046
Haploinsufficiency of Adenomatous Polyposis Coli Coupled with Kirsten Rat Sarcoma Viral Oncogene Homologue Activation and P53 Loss Provokes High-Grade Glioblastoma Formation in Mice
Abstract
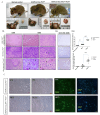
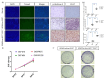
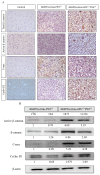
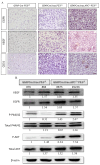
Glioblastoma multiforme (GBM) is the most common and deadly type of brain tumor originating from glial cells. Despite decades of clinical trials and research, there has been limited success in improving survival rates. However, molecular pathology studies have provided a detailed understanding of the genetic alterations associated with the formation and progression of glioblastoma-such as Kirsten rat sarcoma viral oncogene homolog (KRAS) signaling activation (5%), P53 mutations (25%), and adenomatous polyposis coli (APC) alterations (2%)-laying the groundwork for further investigation into the biological and biochemical basis of this malignancy. These analyses have been crucial in revealing the sequential appearance of specific genetic lesions at distinct histopathological stages during the development of GBM. To further explore the pathogenesis and progression of glioblastoma, here, we developed the glial-fibrillary-acidic-protein (GFAP)-Cre-driven mouse model and demonstrated that activated KRAS and p53 deficiencies play distinct and cooperative roles in initiating glioma tumorigenesis. Additionally, the combination of APC haploinsufficiency with mutant Kras activation and p53 deletion resulted in the rapid progression of GBM, characterized by perivascular inflammation, large necrotic areas, and multinucleated giant cells. Consequently, our GBM models have proven to be invaluable resources for identifying early disease biomarkers in glioblastoma, as they closely mimic the human disease. The insights gained from these models may pave the way for potential advancements in the diagnosis and treatment of this challenging brain tumor.
Keywords: APC haploinsufficiency; animal models; giant cells; glioblastoma multiforme (GBM).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Evolutionary etiology of high-grade astrocytomas.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):17933-8. doi: 10.1073/pnas.1317026110. Epub 2013 Oct 10. Proc Natl Acad Sci U S A. 2013. PMID: 24114272 Free PMC article.
-
Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme.Tumour Biol. 2013 Aug;34(4):2063-74. doi: 10.1007/s13277-013-0871-3. Epub 2013 Jun 5. Tumour Biol. 2013. PMID: 23737287 Review.
-
[Neuregulin 2 is highly expressed in glioma tissues to regulate glial fibrillary acidic protein expression via Akt signaling].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Aug 20;41(8):1171-1176. doi: 10.12122/j.issn.1673-4254.2021.08.07. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34549707 Free PMC article. Chinese.
-
Detection of p53 mutations in proliferating vascular cells in glioblastoma multiforme.J Neurosurg. 2015 Feb;122(2):317-23. doi: 10.3171/2014.10.JNS132159. Epub 2014 Nov 21. J Neurosurg. 2015. PMID: 25415071
-
Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis.Adv Biol Regul. 2018 May;68:46-54. doi: 10.1016/j.jbior.2018.01.001. Epub 2018 Jan 10. Adv Biol Regul. 2018. PMID: 29449169 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

