Combined Antitumor Effect of the Serine Protease Urokinase Inhibitor Upamostat and the Sphingosine Kinase 2 Inhibitor Opaganib on Cholangiocarcinoma Patient-Derived Xenografts
- PMID: 38473407
- PMCID: PMC10930726
- DOI: 10.3390/cancers16051050
Combined Antitumor Effect of the Serine Protease Urokinase Inhibitor Upamostat and the Sphingosine Kinase 2 Inhibitor Opaganib on Cholangiocarcinoma Patient-Derived Xenografts
Abstract
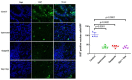
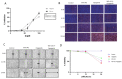
Upamostat is an orally available small-molecule serine protease inhibitor that is a highly potent inhibitor of trypsin 1, trypsin 2, trypsin 3 (PRSS1/2/3), and the urokinase-type plasminogen activator (uPA). These enzymes are expressed in many cancers, especially during tissue remodeling and subsequent tumor cell invasion. Opaganib (ABC294640), a novel, orally available small molecule is a selective inhibitor of the phosphorylation of sphingosine to sphingosine-1-phosphate (S-1-P) by sphingosine kinase 2 (SPHK2). Both sphingosine kinase 1 (SPHK1) and SPHK2 are known to regulate the proliferation-inducing compound S-1-P. However, SPHK2 is more critical in cancer pathogenesis. The goal of this project was to investigate the potential antitumor effects of upamostat and opaganib, individually and in combination, on cholangiocarcinoma (CCA) xenografts in nude mice. PAX165, a patient-derived xenograft (PDX) from a surgically resected CCA, expresses substantial levels of SPHK2, PRSS1, PRSS2, and PRSS3. Four groups of 18 mice each were treated with upamostat, opaganib, both, or vehicle. Mouse weights and PAX165 tumor volumes were measured. Tumor volumes in the upamostat, opaganib, and upamostat plus opaganib groups were significantly decreased compared to the control group.
Keywords: WX-UK1; cholangiocellular carcinoma; opaganib; patient-derived xenograft (PDX); serine protease; sphingosine kinase; upamostat.
Conflict of interest statement
The authors Vered Katz Ben-Yair, Mark L. Levitt, and Reza Fathi were paid consultants for RedHill Biopharma, Ltd. when the data for this study were generated. Lewis R. Roberts has received grant support from Bayer, Boston Scientific, Exact Sciences, Gilead Sciences, Glycotest, RedHill Biopharma, Target Real World Evidence, and Fujifilm; he has provided advisory services to Bayer, Exact Sciences, Gilead Sciences, and GRAIL. The other authors have no conflicts of interest to declare.
Figures





References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. [(accessed on 24 April 2023)];CA Cancer J. Clin. 2021 71:209–249. doi: 10.3322/caac.21660. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/epdf/10.3322/caac.21660. - DOI - PubMed
-
- Valle J.W., Lamarca A., Goyal L., Barriuso J., Zhu A.X. REVIEW|New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943–962. doi: 10.1158/2159-8290.CD-17-0245. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

