Impact of Primary Tumor Location on Demographics, Resectability, Outcomes, and Quality of Life in Finnish Metastatic Colorectal Cancer Patients (Subgroup Analysis of the RAXO Study)
- PMID: 38473410
- PMCID: PMC10931274
- DOI: 10.3390/cancers16051052
Impact of Primary Tumor Location on Demographics, Resectability, Outcomes, and Quality of Life in Finnish Metastatic Colorectal Cancer Patients (Subgroup Analysis of the RAXO Study)
Abstract
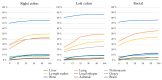
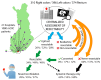
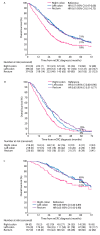
The primary tumor location (PTL) is associated with the phenotype, metastatic sites, mutations, and outcomes of metastatic colorectal cancer (mCRC) patients, but this has mostly been studied according to sidedness (right vs. left sided). We studied right colon vs. left colon vs. rectal PTL in a real-life study population (n = 1080). Health-related quality of life (HRQoL) was assessed multi-cross-sectionally with QLQ-C30, QLQ-CR29, EQ-5D, and 15D. A chi-square, Kaplan-Meier, and Cox regression were used to compare the groups. The PTL was in the right colon in 310 patients (29%), the left colon in 396 patients (37%), and the rectum in 375 patients (35%). The PTL was associated with distinct differences in metastatic sites during the disease trajectory. The resectability, conversion, and resection rates were lowest in the right colon, followed by the rectum, and were highest in the left colon. Overall survival was shortest for right colon compared with left colon or rectal PTL (median 21 vs. 35 vs. 36 months), with the same trends after metastasectomy or systemic therapy only. PTL also remained statistically significant in a multivariable model. The distribution of symptoms varied according to PTL, especially between the right colon (with general symptoms of metastases) and rectal PTL (with sexual- and bowel-related symptoms). mCRC, according to PTL, behaves differently regarding metastatic sites, resectability of the metastases, outcomes of treatment, and HRQoL.
Keywords: metastasectomy; metastatic colorectal cancer; primary tumor location; quality of life; resectability.
Conflict of interest statement
All authors report institutional research funding from Eli Lilly, Merck KGaA, Roche Finland, Sanofi, and unrestricted grants from Amgen and Servier, during the conduct of the study. S.A., E.O., A.R., L.N., J.S., M.J.M., T.K., S.K., A.Å., R.R., E.H., R.K., P.H., L.-M.S., A.N., A.U., T.S., H.S., A.L., T.M., J.K., B.G., H.I., K.L. and P.O. report grants, personal fees, or non-financial support from AbbVie, Amgen, Agenus, Astellas, Astra- Zeneca, Baxalta/Shire, Bayer, BMS, Celgene, Eisai/Ewopharma, Eli Lilly, Erythech Pharma, Fresenius, Incyte, Jansen-Cilag, Medicom, Merck, MSD, Nordic Drugs/Pharma, Novartis, Nutricia/Danone, Pierre-Fabre, Roche, Sanofi, Servier, Sobi, Takeda, and/or Varian.
Figures


Similar articles
-
Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study.Cancers (Basel). 2022 Mar 28;14(7):1713. doi: 10.3390/cancers14071713. Cancers (Basel). 2022. PMID: 35406485 Free PMC article.
-
Understanding the Prognostic Value of Primary Tumor Location and KRAS in Metastatic Colorectal Cancer: A Post Hoc Analysis of the OPTIMOX3 DREAM Phase III Study.Clin Colorectal Cancer. 2020 Sep;19(3):200-208.e1. doi: 10.1016/j.clcc.2020.02.012. Epub 2020 Mar 6. Clin Colorectal Cancer. 2020. PMID: 32303437 Clinical Trial.
-
Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study).J Clin Med. 2023 May 18;12(10):3541. doi: 10.3390/jcm12103541. J Clin Med. 2023. PMID: 37240646 Free PMC article.
-
The primary tumor location in colorectal cancer: A focused review on its impact on surgical management.Glob Health Med. 2021 Dec 31;3(6):386-393. doi: 10.35772/ghm.2020.01096. Glob Health Med. 2021. PMID: 35036620 Free PMC article. Review.
-
Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis.Cancer Med. 2020 Feb;9(3):1044-1057. doi: 10.1002/cam4.2747. Epub 2019 Dec 19. Cancer Med. 2020. PMID: 31856410 Free PMC article.
References
-
- Arnold D., Lueza B., Douillard J.Y., Peeters M., Lenz H.J., Venook A., Heinemann V., Van Cutsem E., Pignon J.P., Tabernero J., et al. Prognostic and Predictive Value of Primary Tumour Side in Patients with RAS Wild-Type Metastatic Colorectal Cancer Treated with Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann. Oncol. 2017;28:1713–1729. doi: 10.1093/annonc/mdx175. - DOI - PMC - PubMed
-
- Venook A.P., Niedzwiecki D., Innocenti F., Fruth B., Greene C., O’Neil B.H., Shaw J.E., Atkins J.N., Horvath L.E., Polite B.N., et al. Impact of Primary (1°) Tumor Location on Overall Survival (OS) and Progression-Free Survival (PFS) in Patients (Pts) with Metastatic Colorectal Cancer (MCRC): Analysis of CALGB/SWOG 80405 (Alliance) J. Clin. Oncol. 2016;34:3504. doi: 10.1200/JCO.2016.34.15_suppl.3504. - DOI
Grants and funding
- 2016, 2018, 2019, 2020, 2021, 2022, 2023/Finska Läkaresällskapet
- 2019-2020, 2021, 2022-2023/Finnish Cancer Registry
- 2020-2022/Relanderin säätiö
- 2012, 2016, 2017, 2018, 2019, 2020, 2021, 2022, 2023/Competitive State Research Financing of the Expert Responsibility Area of Tampere, Helsinki, Turku, Kuopio, Oulu, and Satakunta Hospitals
- Tukisäätiö 2019, 2020, 2023 and OOO-project 2020/Tampere University Hospital
LinkOut - more resources
Full Text Sources

