New Biodegradable Copolymers Based on Betulin and Hydroxycarboxylic Acid Derivatives
- PMID: 38473454
- PMCID: PMC10934601
- DOI: 10.3390/ma17050981
New Biodegradable Copolymers Based on Betulin and Hydroxycarboxylic Acid Derivatives
Abstract
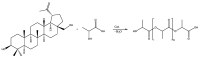
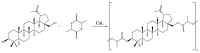
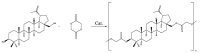
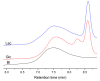
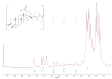
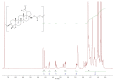
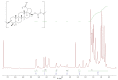
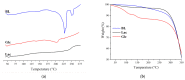
In this study, we propose an approach to the synthesis of new biodegradable polymer materials based on renewable raw feedstock (betulin) and derivatives of hydroxycarboxylic acids using a catalyst/catalytic system (γ-Al2O3, γ-Al2O3/TBHP) that is safe for health and the environment. The resulting polymers are linear thermoplastic polymers that undergo collapse upon melting in the presence of atmospheric oxygen. Moreover, these polymers demonstrate non-toxicity towards a range of Gram-positive and Gram-negative bacteria. The polycondensation of betulin with butyl lactate is particularly noteworthy.
Keywords: ROP; betulin; biodegradable polymers; polycondensation; renewable raw materials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Biodegradable and Bioactive Carriers Based on Poly(betulin disuccinate-co-sebacic Acid) for Rifampicin Delivery.Pharmaceutics. 2022 Mar 6;14(3):579. doi: 10.3390/pharmaceutics14030579. Pharmaceutics. 2022. PMID: 35335954 Free PMC article.
-
Highly Branched Betulin Based Polyanhydrides for Self-Assembled Micellar Nanoparticles Formulation.Int J Mol Sci. 2022 Sep 28;23(19):11462. doi: 10.3390/ijms231911462. Int J Mol Sci. 2022. PMID: 36232762 Free PMC article.
-
Towards lignin derived thermoplastic polymers.Int J Biol Macromol. 2020 Dec 15;165(Pt B):3180-3197. doi: 10.1016/j.ijbiomac.2020.09.173. Epub 2020 Oct 13. Int J Biol Macromol. 2020. PMID: 33065157 Review.
-
Intrinsically microporous polyesters from betulin - toward renewable materials for gas separation made from birch bark.Macromol Rapid Commun. 2011 Nov 15;32(22):1846-51. doi: 10.1002/marc.201100532. Epub 2011 Sep 19. Macromol Rapid Commun. 2011. PMID: 21928305
-
Biotechnological production and high potential of furan-based renewable monomers and polymers.Biotechnol Adv. 2021 May-Jun;48:107707. doi: 10.1016/j.biotechadv.2021.107707. Epub 2021 Feb 22. Biotechnol Adv. 2021. PMID: 33631186 Review.
References
-
- Tolstikov G.A., Flekhter O.B., Shultz E.E., Baltina L.A., Tolstikov A.G. Betulin and Its Derivatives. Chemistry and Biological Activity. Chem. Sustain. Dev. 2005;13:1–29.
-
- Jonnalagadda S.C., Suman P., Morgan D.C., Seay J.N. Studies in Natural Products Chemistry. Volume 53. Elsevier B.V.; Amsterdam, The Netherlands: 2017. Recent Developments on the Synthesis and Applications of Betulin and Betulinic Acid Derivatives as Therapeutic Agents; pp. 45–84.
-
- Kislitsyn A.N. Birch Bark Extractives: Isolation, Composition, Properties, Application. Chem. Wood. 1994;3:325–341. (In Russian)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

