Improving the Corrosion Performance of Organically Coated Steel Using a Sol-Gel Overcoat
- PMID: 38473547
- PMCID: PMC10934472
- DOI: 10.3390/ma17051075
Improving the Corrosion Performance of Organically Coated Steel Using a Sol-Gel Overcoat
Abstract
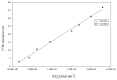
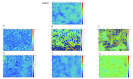
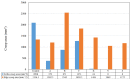
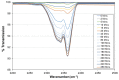
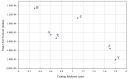
Organically coated steels are widely used in applications in which they are subjected to the natural environment and therefore require excellent corrosion resistance. Organic clearcoats are typically employed as a barrier that improves the overall corrosion resistance; however, they are typically derived from fossil fuel-based feedstock. A more sustainable alternative could be possible using sol-gel coatings. The application of a simple tetraethoxysilane (TEOS)-based sol-gel was applied to polyurethane-coated steels using a spray coater. The concentration of TEOS was altered to produce coatings containing either 2.5% or 10%. The 10% TEOS resulted in dense, homogeneous coatings that offered a significant improvement in corrosion resistance compared to an uncoated substrate. Whereas the 2.5% TEOS coatings were inhomogeneous and porous, which indicated a limitation of concentration required to produce a uniform coating. The successful demonstration of using a simple TEOS-based coating to improve the corrosion resistance of organically coated steel highlights the potential for further investigation into the use of sol-gels for these applications.
Keywords: barrier; coatings; coil coating; corrosion; sol–gel.
Conflict of interest statement
Author Peter Barker was employed by Tata Steel UK, Shotton Works. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Sander J. Coil Coating. Vincentz Network; Hanover, Germany: 2014.
-
- Knudsen O.O., Forsgren A. Corrosion Control Through Organic Coatings. 2nd ed. CRC Press; Boca Raton, FL, USA: 2017.
-
- Morcillo M., Rodríguez F., Bastidas J. The influence of chlorides, sulphates and nitrates at the coating-steel interface on underfilm corrosion. Prog. Org. Coat. 1997;31:245–253. doi: 10.1016/S0300-9440(97)00083-0. - DOI
-
- Zhang J.T., Hu J.M., Zhang J.Q., Cao C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004;49:293–301. doi: 10.1016/S0300-9440(03)00115-2. - DOI
-
- Fredj N., Cohendoz S., Mallarino S., Feaugas X., Touzain S. Evidencing antagonist effects of water uptake and leaching processes in marine organic coatings by gravimetry and EIS. Prog. Org. Coat. 2010;67:287–295. doi: 10.1016/j.porgcoat.2009.11.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

