Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye
- PMID: 38473569
- PMCID: PMC10933863
- DOI: 10.3390/ma17051097
Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye
Abstract
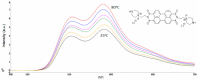
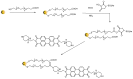
We developed a fluorescent molecular probe based on gold nanoparticles functionalized with N,N'-bis(2-(1-piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic acid diimide dihydrochloride, and these probes exhibit potential for applications in microscopic thermometry. The intensity of fluorescence was affected by changes in temperature. Chemical environments, such as different buffers with the same pH, also resulted in different fluorescence intensities. Due to the fluorescence intensity changes exhibited by modified gold nanoparticles, these materials are promising candidates for future technologies involving microscopic temperature measurements.
Keywords: gold nanoparticles; gold nanorods; perylene-derivative dye; temperature-dependent fluorescence.
Conflict of interest statement
The authors, A.L., J.D., A.M., A.D. and P.B. are employed by the company ProChimia Surfaces Sp. z o.o. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
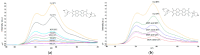
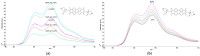






Similar articles
-
Temperature and chemical environment effects on the aggregation extent of water soluble perylene dye into vinyl alcohol-containing polymers.Phys Chem Chem Phys. 2009 Aug 7;11(29):6276-82. doi: 10.1039/b903120k. Epub 2009 May 20. Phys Chem Chem Phys. 2009. PMID: 19606340
-
N-(2-(N',N'-diethylamino)ethyl)perylene-3,4-dicarboximide and its quaternized derivatives as fluorescence probes of acid, temperature, and solvent polarity.J Fluoresc. 2011 Jan;21(1):213-22. doi: 10.1007/s10895-010-0708-z. Epub 2010 Aug 25. J Fluoresc. 2011. PMID: 20737285
-
A water-soluble fluorescent pH probe based on perylene dyes and its application to cell imaging.Luminescence. 2016 Feb;31(1):102-7. doi: 10.1002/bio.2930. Epub 2015 May 26. Luminescence. 2016. PMID: 26009881
-
Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation.Sci Total Environ. 2021 Aug 1;780:146483. doi: 10.1016/j.scitotenv.2021.146483. Epub 2021 Mar 17. Sci Total Environ. 2021. PMID: 33773344 Review.
-
Functionalized silica nanoparticles: a platform for fluorescence imaging at the cell and small animal levels.Acc Chem Res. 2013 Jul 16;46(7):1367-76. doi: 10.1021/ar3001525. Epub 2013 Mar 14. Acc Chem Res. 2013. PMID: 23489227 Review.
Cited by
-
Monte Carlo Simulations in Nanomedicine: Advancing Cancer Imaging and Therapy.Nanomaterials (Basel). 2025 Jan 15;15(2):117. doi: 10.3390/nano15020117. Nanomaterials (Basel). 2025. PMID: 39852732 Free PMC article. Review.
References
-
- Ogle M.M., Smith McWilliams A.D., Jiang B., Martí A.A. Latest trends in temperature sensing by molecular probes. ChemPhotoChem. 2020;4:255–270. doi: 10.1002/cptc.201900255. - DOI
-
- Su S., Wei J., Zhang K., Qiu J., Wang S. Thermo- and pH responsive fluorescence behaviors of sulfur-functionalized detonation nanodiamond-poly(N-isopropylacrylamide) Colloid Polym. Sci. 2015;293:1299–1305. doi: 10.1007/s00396-015-3531-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

