Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations
- PMID: 38473622
- PMCID: PMC10934206
- DOI: 10.3390/ma17051151
Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations
Abstract
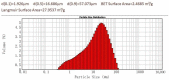
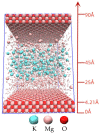
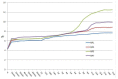
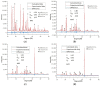
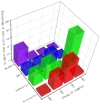
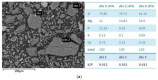
Magnesium potassium phosphate cement (MKPC) is formed on the basis of acid-base reaction between dead burnt MgO and KH2PO4 in aqueous solution with K-struvite as the main cementitious phase. Due to the unique characteristics of these cements, they are suitable for special applications, especially the immobilization of radioactive metal cations and road repair projects at low temperature. However, there are few articles about the hydration mechanism of MKPC. In this study, the types, proportions and formation mechanism of MKPC crystalline phases under different magnesium to phosphorus (Mg/P) ratios were studied by means of AAS, ICP-OES, SEM, EDS and XRD refinement methods. Corresponding MD simulation works were used to explain the hydration mechanism. This study highlights the fact that crystalline phases distribution of MKPC could be adjusted and controlled by different Mg/P ratios for the design of the MKPC, and the key factor is the kinetic of K+.
Keywords: K-struvite; diffusion kinetics of K+; magnesium potassium phosphate cement; newberyite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wilson A.D. The chemistry of dental cements. Chem. Soc. Rev. 1978;7:265–296. doi: 10.1039/cs9780700265. - DOI
-
- Kingery W.D. Fundamental Study of Phosphate Bonding in Refractories: I, Literature Review. J. Am. Ceram. Soc. 1950;33:239–241. doi: 10.1111/j.1151-2916.1950.tb14171.x. - DOI
-
- Gardner L.J., Walling S.A., Corkhill C.L., Bernal S.A., Lejeune V., Stennett M.C., Provis J.L., Hyatt N.C. Temperature transformation of blended magnesium potassium phosphate cement binders. Cem. Concr. Res. 2021;141:106332. doi: 10.1016/j.cemconres.2020.106332. - DOI
-
- Wagh A.S., Jeong S.Y. Chemically Bonded Phosphate Ceramics: I, A Dissolution Model of Formation. J. Am. Ceram. Soc. 2003;86:1838–1844. doi: 10.1111/j.1151-2916.2003.tb03569.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

