Molecular Serum Albumin Unmask Nanobio Properties of Molecular Graphenes in Shungite Carbon Nanoparticles
- PMID: 38473711
- PMCID: PMC10930998
- DOI: 10.3390/ijms25052465
Molecular Serum Albumin Unmask Nanobio Properties of Molecular Graphenes in Shungite Carbon Nanoparticles
Abstract
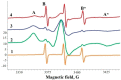
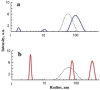
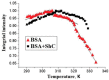
Serum albumin is a popular macromolecule for studying the effect of proteins on the colloidal stability of nanoparticle (NP) dispersions, as well as the protein-nanoparticle interaction and protein corona formation. In this work, we analyze the specific conformation-dependent phase, redox, and fatty acid delivery properties of bovine albumin in the presence of shungite carbon (ShC) molecular graphenes stabilized in aqueous dispersions in the form of NPs in order to reveal the features of NP bioactivity. The formation of NP complexes with proteins (protein corona around NP) affects the transport properties of albumin for the delivery of fatty acids. Being acceptors of electrons and ligands, ShC NPs are capable of exhibiting both their own biological activity and significantly affecting conformational and phase transformations in protein systems.
Keywords: fatty acids; metastable clusters; protein corona; redox bioactivity; serum albumin; shungite carbon nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
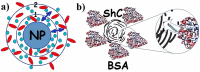




Similar articles
-
Fatty acid transfer between serum albumins and shungite carbon nanoparticles and its effect on protein aggregation and association.Eur Biophys J. 2020 Jan;49(1):85-94. doi: 10.1007/s00249-019-01414-y. Epub 2019 Dec 21. Eur Biophys J. 2020. PMID: 31865396
-
Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes.Acc Chem Res. 2014 Aug 19;47(8):2651-9. doi: 10.1021/ar500190q. Epub 2014 Jul 11. Acc Chem Res. 2014. PMID: 25014679 Free PMC article.
-
Different Serum, Different Protein Corona! The Impact of the Serum Source on Cellular Targeting of Folic Acid-Modified Chitosan-Based Nanoparticles.Mol Pharm. 2022 May 2;19(5):1635-1646. doi: 10.1021/acs.molpharmaceut.2c00108. Epub 2022 Apr 5. Mol Pharm. 2022. PMID: 35380849
-
A health concern regarding the protein corona, aggregation and disaggregation.Biochim Biophys Acta Gen Subj. 2019 May;1863(5):971-991. doi: 10.1016/j.bbagen.2019.02.012. Epub 2019 Feb 22. Biochim Biophys Acta Gen Subj. 2019. PMID: 30802594 Free PMC article. Review.
-
Albumin corona on nanoparticles - a strategic approach in drug delivery.Drug Deliv. 2016 Oct;23(8):2668-2676. doi: 10.3109/10717544.2015.1048488. Epub 2015 Jun 9. Drug Deliv. 2016. PMID: 26056719 Review.
Cited by
-
Stable Nitroxide as Diagnostic Tools for Monitoring of Oxidative Stress and Hypoalbuminemia in the Context of COVID-19.Int J Mol Sci. 2024 Jul 24;25(15):8045. doi: 10.3390/ijms25158045. Int J Mol Sci. 2024. PMID: 39125614 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

