Knockdown of DNMT1 Induces SLCO3A1 to Promote Follicular Growth by Enhancing the Proliferation of Granulosa Cells in Mammals
- PMID: 38473715
- PMCID: PMC10930803
- DOI: 10.3390/ijms25052468
Knockdown of DNMT1 Induces SLCO3A1 to Promote Follicular Growth by Enhancing the Proliferation of Granulosa Cells in Mammals
Abstract
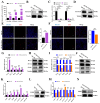
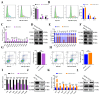
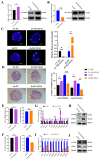
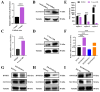
In female mammals, the proliferation and apoptosis of granulosa cells (GCs) have been shown to determine the fate of follicles. DNA methyltransferases (DNMTs) and SLCO3A1 have been reported to be involved in the survival of GCs and follicular growth. However, the molecular mechanisms enabling DNMTs to regulate the expression of SLCO3A1 to participate in follicular growth are unclear. In this study, we found that the knockdown of DNMT1 enhanced the mRNA and protein levels of SLCO3A1 by regulating the chromatin accessibility probably. Moreover, SLCO3A1 upregulated the mRNA and protein levels of MCL1, PCNA, and STAR to promote the proliferation of GCs and facilitated cell cycle progression by increasing the mRNA and protein levels of CCNE1, CDK2, and CCND1, but it decreased apoptosis by downregulating the mRNA and protein levels of CASP3 and CASP8. Moreover, SLCO3A1 promoted the growth of porcine follicles and development of mice follicles. In conclusion, the knockdown of DNMT1 upregulated the mRNA and protein levels of SLCO3A1, thereby promoting the proliferation of GCs to facilitate the growth and development of ovarian follicles, and these results provide new insights into investigations of female reproductive diseases.
Keywords: DNMT1; SLCO3A1; follicular growth; proliferation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
FGA, a new target of histone acetylation, inhibits apoptosis of granulosa cells in follicles.Biol Res. 2025 Jul 2;58(1):43. doi: 10.1186/s40659-025-00623-4. Biol Res. 2025. PMID: 40605115 Free PMC article.
-
DNA methylation mediated RSPO2 to promote follicular development in mammals.Cell Death Dis. 2021 Jun 26;12(7):653. doi: 10.1038/s41419-021-03941-z. Cell Death Dis. 2021. PMID: 34175894 Free PMC article.
-
P65 Targets FGFR1 to Regulate the Survival of Ovarian Granulosa Cells.Cells. 2019 Oct 29;8(11):1334. doi: 10.3390/cells8111334. Cells. 2019. PMID: 31671754 Free PMC article.
-
Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway.Molecules. 2021 Sep 16;26(18):5635. doi: 10.3390/molecules26185635. Molecules. 2021. PMID: 34577105 Free PMC article.
-
Ovarian follicular growth and atresia: the relationship between cell proliferation and survival.J Anim Sci. 2004;82 E-Suppl:E40-52. doi: 10.2527/2004.8213_supplE40x. J Anim Sci. 2004. PMID: 15471814 Review.
Cited by
-
DNA Methylation Mediates the Transcription of STAT4 to Regulate KISS1 During Follicular Development.Cells. 2025 Apr 1;14(7):523. doi: 10.3390/cells14070523. Cells. 2025. PMID: 40214477 Free PMC article.
References
-
- Murase T., Iwase A., Komatsu K., Bayasula, Nakamura T., Osuka S., Takikawa S., Goto M., Kotani T., Kikkawa F. Follicle Dynamics: Visualization and Analysis of Follicle Growth and Maturation Using Murine Ovarian Tissue Culture. J. Assist. Reprod. Genet. 2018;35:339–343. doi: 10.1007/s10815-017-1073-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2023A1515030054/Guangdong Basic and Applied Basic Research Foundation
- 2023A1515010364/Guangdong Basic and Applied Basic Research Foundation
- 31902131/National Natural Science Foundation of China
- 32072694/National Natural Science Foundation of China
- CARS-35/Earmarked fund for China Agriculture Research System
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

