Immunological Landscapes in Lung Transplantation: Insights from T Cell Profiling in BAL and PBMC
- PMID: 38473722
- PMCID: PMC10931512
- DOI: 10.3390/ijms25052476
Immunological Landscapes in Lung Transplantation: Insights from T Cell Profiling in BAL and PBMC
Abstract
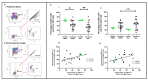
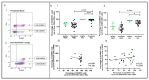
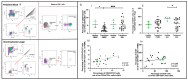
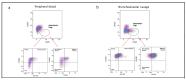
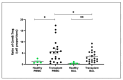
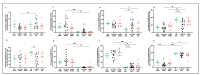
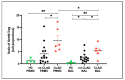
Lung transplant recipients frequently encounter immune-related complications, including chronic lung allograft dysfunction (CLAD). Monitoring immune cells within the lung microenvironment is pivotal for optimizing post-transplant outcomes. This study examined the proportion of T cell subsets in paired bronchoalveolar lavage (BAL) and peripheral PBMC comparing healthy (n = 4) and lung transplantation patients (n = 6, no CLAD and n = 14 CLAD) using 14-color flow cytometry. CD4+ T cell proportions were reduced in CD3 cells in both PBMC and BAL, and positive correlations were discerned between T cell populations in peripheral PBMC and BAL, suggesting the prospect of employing less invasive PBMC sampling as a means of monitoring lung T cells. Furthermore, regulatory T cells (Tregs) were enriched in BAL when compared to peripheral PBMC for transplant recipients. A parallel positive correlation emerged between Treg proportions in BAL and peripheral PBMC, underscoring potential avenues for monitoring lung Tregs. Finally, the most promising biomarker was the Teff (CD8+Granzyme B+)-Treg ratio, which was higher in both the PBMC and BAL of transplant recipients compared to healthy individuals, and increased in the patients with CLAD compared to no CLAD and healthy patients. Conclusions: Distinct T cell profiles in BAL and peripheral PBMC underscore the significance of localized immune monitoring in lung transplantation. The Teff (CD8+granzyme B+)-Treg ratio, particularly within the context of CLAD, emerges as a promising blood and BAL biomarker reflective of inflammation and transplant-related complications. These findings emphasize the imperative need for personalized immune monitoring strategies that tailored to address the unique immunological milieu in post-transplant lungs.
Keywords: BAL; CLAD; PBMC; Tregs; granzyme B; lung transplant.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Chambers D.C., Cherikh W.S., Goldfarb S.B., Hayes D., Kucheryavaya A.Y., Toll A.E., Khush K.K., Levvey B.J., Meiser B., Rossano J.W., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018;37:1169–1183. doi: 10.1016/j.healun.2018.07.020. - DOI - PubMed
-
- Glanville A.R., Verleden G.M., Todd J.L., Benden C., Calabrese F., Gottlieb J., Hachem R.R., Levine D., Meloni F., Palmer S.M., et al. Chronic lung allograft dysfunction: Definition update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019;38:483–492. doi: 10.1016/j.healun.2019.03.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

