Impact of Immunopathy and Coagulopathy on Multi-Organ Failure and Mortality in a Lethal Porcine Model of Controlled and Uncontrolled Hemorrhage
- PMID: 38473750
- PMCID: PMC10931034
- DOI: 10.3390/ijms25052500
Impact of Immunopathy and Coagulopathy on Multi-Organ Failure and Mortality in a Lethal Porcine Model of Controlled and Uncontrolled Hemorrhage
Abstract
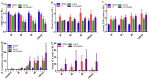
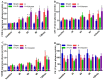
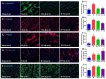
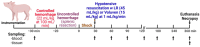
Uncontrolled hemorrhage is a major preventable cause of death in patients with trauma. However, the majority of large animal models of hemorrhage have utilized controlled hemorrhage rather than uncontrolled hemorrhage to investigate the impact of immunopathy and coagulopathy on multi-organ failure (MOF) and mortality. This study evaluates these alterations in a severe porcine controlled and uncontrolled hemorrhagic shock (HS) model. Anesthetized female swine underwent controlled hemorrhage and uncontrolled hemorrhage by partial splenic resection followed with or without lactated Ringer solution (LR) or Voluven® resuscitation. Swine were surveyed 6 h after completion of splenic hemorrhage or until death. Blood chemistry, physiologic variables, systemic and tissue levels of complement proteins and cytokines, coagulation parameters, organ function, and damage were recorded and assessed. HS resulted in systemic and local complement activation, cytokine release, hypocoagulopathy, metabolic acidosis, MOF, and no animal survival. Resuscitation with LR and Voluven® after HS improved hemodynamic parameters (MAP and SI), metabolic acidosis, hyperkalemia, and survival but resulted in increased complement activation and worse coagulopathy. Compared with the LR group, the animals with hemorrhagic shock treated with Voluven® had worse dilutional anemia, coagulopathy, renal and hepatic dysfunction, increased myocardial complement activation and renal damage, and decreased survival rate. Hemorrhagic shock triggers early immunopathy and coagulopathy and appears associated with MOF and death. This study indicates that immunopathy and coagulopathy are therapeutic targets that may be addressed with a high-impact adjunctive treatment to conventional resuscitation.
Keywords: MOF; coagulopathy; damage control resuscitation; immunopathy; mortality; swine; uncontrolled hemorrhagic shock.
Conflict of interest statement
The authors declare no conflicts of interest relevant to the manuscript submitted to the International Journal of Molecular Sciences. The opinions or assertions contained herein are the private views of the authors, and they are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Figures

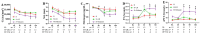


Similar articles
-
Lactated Ringer's is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock.J Trauma. 2007 Mar;62(3):636-9. doi: 10.1097/TA.0b013e31802ee521. J Trauma. 2007. PMID: 17414340
-
Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer's solution.J Trauma. 2006 Jan;60(1):52-63. doi: 10.1097/01.ta.0000200156.05397.0b. J Trauma. 2006. PMID: 16456436
-
Three- versus four-factor prothrombin complex concentrates for "factor-based" resuscitation in a porcine hemorrhagic shock model.J Trauma Acute Care Surg. 2017 Dec;83(6):1114-1123. doi: 10.1097/TA.0000000000001646. J Trauma Acute Care Surg. 2017. PMID: 28700408
-
Traumatic coagulopathy--part 2: Resuscitative strategies.J Vet Emerg Crit Care (San Antonio). 2014 Jan-Feb;24(1):75-92. doi: 10.1111/vec.12138. Epub 2014 Jan 6. J Vet Emerg Crit Care (San Antonio). 2014. PMID: 24393363 Review.
-
Coagulation challenges after severe injury with hemorrhagic shock.J Trauma Acute Care Surg. 2012 Jun;72(6):1714-8. doi: 10.1097/TA.0b013e318245225c. J Trauma Acute Care Surg. 2012. PMID: 22695446 Review.
References
-
- Barea-Mendoza J.A., Chico-Fernández M., Molina-Díaz I., Moreno-Muñoz G., Toboso-Casado J.M., Viña-Soria L., Matachana-Martínez M., Freire-Aragón M.D., Pérez-Bárcena J., Llompart-Pou J.A. Risk Factors Associated with Early and Late Posttraumatic Multiorgan Failure: An Analysis From RETRAUCI. Shock. 2021;55:326–331. doi: 10.1097/SHK.0000000000001628. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

