Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice
- PMID: 38473751
- PMCID: PMC10931593
- DOI: 10.3390/ijms25052503
Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice
Abstract
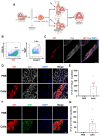
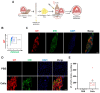
The diaphragm muscle is essential for breathing, and its dysfunctions can be fatal. Many disorders affect the diaphragm, including muscular dystrophies. Despite the clinical relevance of targeting the diaphragm, there have been few studies evaluating diaphragm function following a given experimental treatment, with most of these involving anti-inflammatory drugs or gene therapy. Cell-based therapeutic approaches have shown success promoting muscle regeneration in several mouse models of muscular dystrophy, but these have focused mainly on limb muscles. Here we show that transplantation of as few as 5000 satellite cells directly into the diaphragm results in consistent and robust myofiber engraftment in dystrophin- and fukutin-related protein-mutant dystrophic mice. Transplanted cells also seed the stem cell reservoir, as shown by the presence of donor-derived satellite cells. Force measurements showed enhanced diaphragm strength in engrafted muscles. These findings demonstrate the feasibility of cell transplantation to target the diseased diaphragm and improve its contractility.
Keywords: diaphragm; engraftment; muscle stem cells; muscular dystrophy; pre-injury; regeneration; satellite cells; specific force; transplantation.
Conflict of interest statement
R.C.R.P. is a cofounder of and holds equity in Myogenica. All other authors have no competing financial interests.
Figures


References
-
- Richard I., Hogrel J., Stockholm D., Payan C.A.M., Fougerousse F., Eymard B., Mignard C., de Munain A.L., Fardeau M., Urtizberea J.A., et al. Natural history of LGMD2A for delineating outcome measures in clinical trials. Ann. Clin. Transl. Neurol. 2016;3:248–265. doi: 10.1002/acn3.287. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

