Bisphenol A: Unveiling Its Role in Glioma Progression and Tumor Growth
- PMID: 38473752
- PMCID: PMC10931199
- DOI: 10.3390/ijms25052504
Bisphenol A: Unveiling Its Role in Glioma Progression and Tumor Growth
Abstract
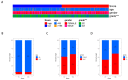
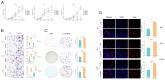
Gliomas represent the most common and lethal category of primary brain tumors. Bisphenol A (BPA), a widely recognized endocrine disruptor, has been implicated in the progression of cancer. Despite its established links to various cancers, the association between BPA and glioma progression remains to be clearly defined. This study aimed to shed light on the impact of BPA on glioma cell proliferation and overall tumor progression. Our results demonstrate that BPA significantly accelerates glioma cell proliferation in a time- and dose-dependent manner. Furthermore, BPA has been found to enhance the invasive and migratory capabilities of glioma cells, potentially promoting epithelial-mesenchymal transition (EMT) characteristics within these tumors. Employing bioinformatics approaches, we devised a risk assessment model to gauge the potential glioma hazards associated with BPA exposure. Our comprehensive analysis revealed that BPA not only facilitates glioma invasion and migration but also inhibits apoptotic processes. In summary, our study offers valuable insights into the mechanisms by which BPA may promote tumorigenesis in gliomas, contributing to the understanding of its broader implications in oncology.
Keywords: bisphenol A (BPA); cell proliferation; glioma; melatonin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Substances
Grants and funding
- 22JR11RA079/Natural Science Foundation of Gansu Province
- 23JRRA0985/Natural Science Foundation of Gansu Province
- 23JRRA1645/Natural Science Foundation of Gansu Province
- CY2021-QN-B03/Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital
- CY2022-QN-A12/Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital
LinkOut - more resources
Full Text Sources

